Deep-sea sugar EPS3.9 sparks immune attack on tumours
Posted: 29 July 2025 | Drug Target Review | No comments yet
Scientists have isolated a sugar molecule from deep-sea bacteria that triggers pyroptosis – a form of inflammatory cell death – to halt tumour growth – highlighting the potential of marine microbes in drug development.
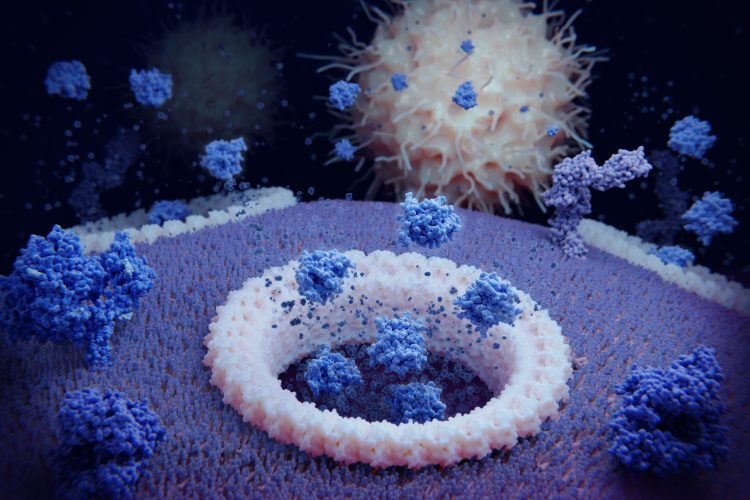

Scientists have discovered a new sugar molecule from deep-sea bacteria that could lead to new cancer therapies. The molecule, called EPS3.9, is an exopolysaccharide – a long-chain sugar – produced by the bacterium Spongiibacter nanhainus CSC3.9. According to the study, published in The FASEB Journal, EPS3.9 promotes pyroptosis – an inflammatory form of programmed cell death – effectively killing tumour cells and suppressing tumour growth.
What is pyroptosis and why does it matter?
Pyroptosis is a type of cell death distinct from apoptosis- characterised by inflammation and the release of signalling molecules that activate the immune system. This inflammatory response can be beneficial in cancer therapy, as it not only eliminates cancer cells but also recruits the body’s defences to attack tumours.
EPS3.9’s ability to induce pyroptosis presents a promising strategy for treating cancers that are resistant to conventional treatments, as it combines direct tumour killing with immune system activation.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
The science behind EPS3.9’s action
Researchers isolated EPS3.9 from the deep ocean and analysed its chemical makeup, identifying mannose and glucose as its primary sugar components. Laboratory experiments demonstrated that EPS3.9 directly targets five specific membrane phospholipids on human leukaemia cells. This interaction triggers pyroptotic cell death- causing the cancer cells to swell and rupture, releasing inflammatory molecules.
Beyond cell cultures, EPS3.9 was tested in mice with liver tumours. The treated animals exhibited significant tumour shrinkage compared to untreated controls. The compound also appeared to stimulate the immune system, suggesting a dual mechanism of tumour suppression.
Implications for future cancer therapies
“Our work not only provides a theoretical basis for developing more carbohydrate-based drugs but also highlights the importance of exploring marine microbial resources,” said Dr Chaomin Sun of the Chinese Academy of Sciences, corresponding author of the study.
The marine environment remains an underexplored reservoir of potentially powerful bioactive compounds. This study highlights how marine microbes can provide new molecules with unique mechanisms of action- such as EPS3.9’s ability to trigger pyroptosis.
Carbohydrate-based drugs are particularly appealing because sugars often exhibit low toxicity and high biocompatibility, making them promising candidates for drug development.
Challenges and next steps
While the findings are promising, EPS3.9 is still in the early stages of research. Scientists will need to investigate its safety profile, effectiveness across different cancer types and optimal delivery methods. Clinical trials will ultimately be required to determine whether EPS3.9 or related compounds can be developed into safe and effective cancer treatments for humans.
By harnessing nature’s biochemical diversity, researchers may be able to develop more effective, targeted treatments that both kill tumours and engage the immune system – potentially improving outcomes for cancer patients.
Related topics
Animal Models, Cancer research, Drug Discovery, Drug Targets, Immuno-oncology, In Vivo, Microbiology, Oncology, Therapeutics, Translational Science
Related conditions
Cancer
Related organisations
the Chinese Academy of Sciences
Related people
Dr Chaomin Sun (the Chinese Academy of Sciences)