Scientists track amyloid plaques in living mice for first time
Posted: 6 October 2025 | Drug Target Review | No comments yet
A new fibre-optic method lets researchers monitor amyloid plaque buildup in living, freely moving mice – offering a minimally invasive way to track Alzheimer’s disease progression and test potential therapies.
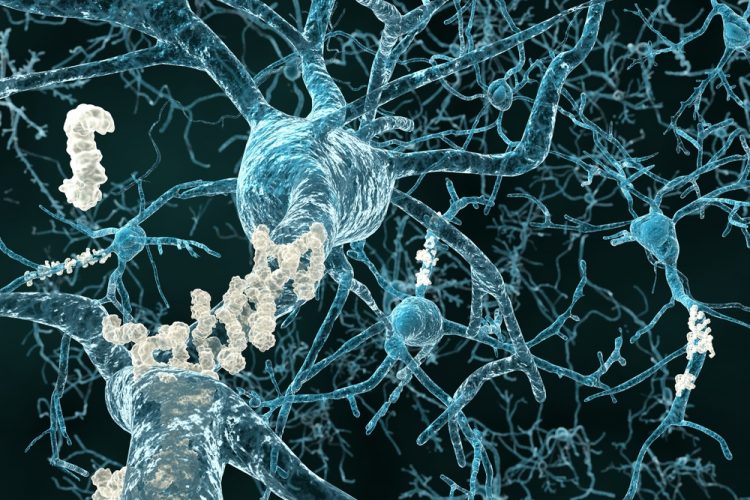

Alzheimer’s disease is characterised by the buildup of amyloid plaques in the brain, but most ways of studying these buildups in mouse models require sacrificing the animals. That limits researchers’ ability to follow how the disease develops or how treatments work over time. However, a new study highlights a fibre-optic method that can monitor plaque signals in living, freely moving mice, enabling easier, more flexible testing of potential therapies.
Adapting fibre photometry to detect amyloid plaques
Researchers at the University of Strathclyde and the Italian Institute of Technology adapted fibre photometry – a technique commonly used to record neural activity – to detect amyloid plaques. Instead of relying on genetically encoded sensors, they used a fluorescent dye called Methoxy-X04 that crosses the blood–brain barrier and binds specifically to amyloid fibrils.
Initial experiments confirm plaque detection
In early experiments, the researchers used flat optical fibres in anaesthetised Alzheimer’s model mice – known as 5xFAD mice – and found that the fluorescence signals matched strongly with plaque density measured afterwards in brain slices. A machine learning model then differentiated between plaque-bearing and healthy animals based on these depth profiles.
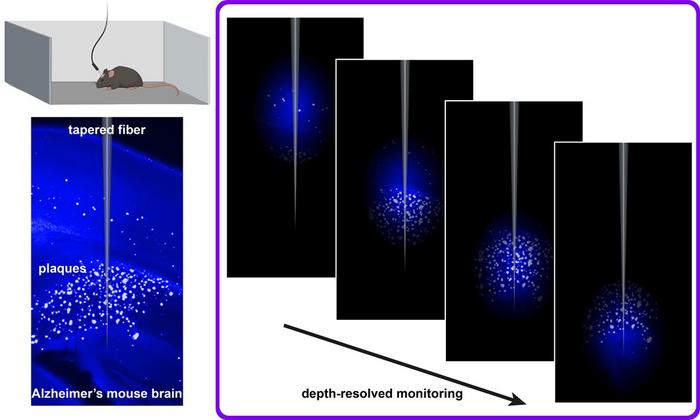

Combining a plaque-binding fluorescent dye with flat and tapered optical fibers enables depth-resolved monitoring across brain regions during natural behavior, offering a minimally invasive way to study disease progression and therapeutic effects in real time. Credit: S. Sakata (University of Strathclyde)
Tapered fibres capture depth-specific signals
Next, the team tested tapered optical fibres, which capture signals from different depths in the brain. In brain tissue slices the tapered fibres reliably tracked plaque distribution. When implanted in living mice, the fibres demonstrated depth-specific increases in fluorescence after being injected with Methoxy-X04 – but only in Alzheimer’s model mice, not in healthy controls. This technique worked in awake, freely moving animals and showed signals increasing with age as the disease progressed.
Advantages over existing imaging methods
Compared with existing methods such as two-photon microscopy or optoacoustic tomography, the new fibre-based approach affords scientists the possibility of long-term monitoring of deep brain regions without anaesthesia. While the method cannot resolve individual plaques, it provides researchers with a minimally invasive way to monitor and track pathological changes across brain regions.
The researchers suggest that this method could help scientists test how potential treatments affect plaque buildup in real time – accelerating the development of new Alzheimer’s therapies.
Related topics
Animal Models, Assays, Central Nervous System (CNS), Imaging, Machine learning, Neurosciences, Translational Science
Related conditions
Alzheimer’s disease
Related organisations
the Italian Institute of Technology, the University of Strathclyde