New nanotherapy clears amyloid-β reversing Alzheimer’s in mice
Posted: 7 October 2025 | Drug Target Review | No comments yet
Researchers have developed bioactive nanoparticles that restore the brain’s blood-brain barrier and clear toxic proteins, reversing Alzheimer’s symptoms in mice and offering a promising new approach to treating the disease.

A new study by a research team co-led by the Institute for Bioengineering of Catalonia (IBEC) and West China Hospital Sichuan University (WCHSU), in collaboration with partners in the UK, have used a nanotechnology strategy that reverses Alzheimer’s disease in mice. Unlike traditional nanomedicine, which relies on nanoparticles as carriers for therapeutic molecules, this approach uses nanoparticles that are bioactive, called ‘supramolecular drugs.’
Instead of targeting neurons directly, this method focuses on repairing the blood-brain barrier (BBB), the brain’s defence against harmful substances. By restoring proper BBB function, the researchers achieved a reversal of Alzheimer’s pathology in animal models.
The importance of brain vasculature
The brain consumes the greatest amount energy out of any other organ in the body, consuming 20 percent in adults and up to 60 percent in children. This energy is delivered through a dense vascular system in which each neuron is nourished by a capillary. With approximately one billion capillaries in the human brain, maintaining vascular health is crucial, particularly in conditions like Alzheimer’s, where vascular function is weakened and linked to disease progression.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
The BBB is a protective barrier between the brain and blood flow, stopping harmful substances such as pathogens and toxins from entering. By targeting specific mechanisms within the BBB, the research team enabled the removal of undesirable waste proteins produced in the brain. In Alzheimer’s disease, the main waste protein is amyloid-β (Aβ), whose accumulation disrupts normal brain function.
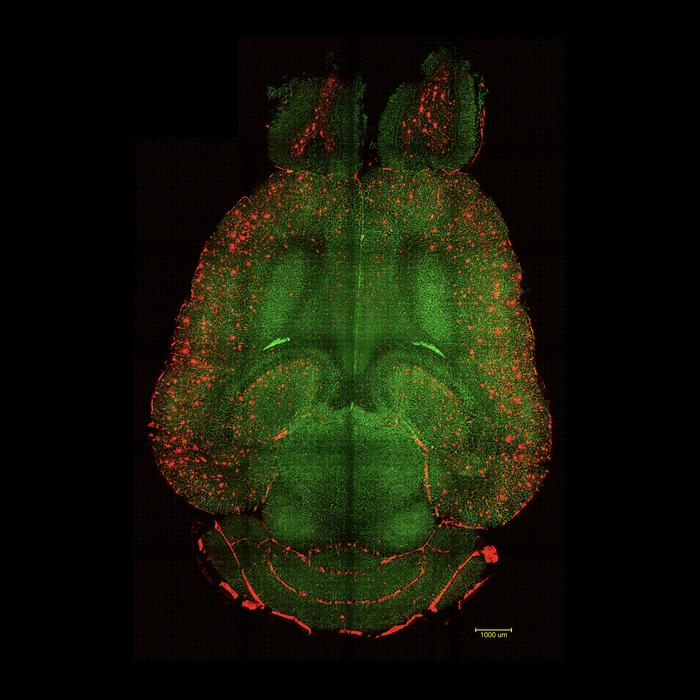

Brain Before: Light sheet fluorescence microscope images of mouse brain 12h after NOT being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
Rapid reduction of amyloid-β
The researchers tested their approach in mice genetically programmed to overproduce Aβ and develop cognitive decline similar to Alzheimer’s pathology. Following only three doses of supramolecular drugs, the team observed significant therapeutic effects.
Following only three doses of supramolecular drugs, the team observed significant therapeutic effects.
“Only 1hr after the injection we observed a reduction of 50-60 percent in Aβ amount inside the brain,” said Junyang Chen, first co-author of the study, researcher at WCHSU and PhD student at University College London.
Behavioural tests conducted over several months demonstrated remarkable improvements. In one experiment, a 12-month-old mouse – equivalent to a 60-year-old human – received the nanoparticles and was evaluated six months later. The animal, now 18 months old – comparable to a 90-year-old human – exhibited the behaviour of a healthy mouse.
Restoring natural clearance mechanisms
“The long-term effect comes from restoring the brain’s vasculature. We think it works like a cascade: when toxic species such as amyloid-beta (Aβ) accumulate, disease progresses. But once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance. What’s remarkable is that our nanoparticles act as a drug and seem to activate a feedback mechanism that brings this clearance pathway back to normal levels,” explained Giuseppe Battaglia, ICREA Research Professor at IBEC and leader of the study.
Normally, the protein LRP1 acts as a molecular gatekeeper, binding to Aβ and transporting it across the BBB for elimination. In Alzheimer’s, this system becomes fragile, leading to Aβ accumulation. The supramolecular drugs mimic LRP1 ligands, binding to Aβ and initiating its clearance, effectively resetting the system and restoring vascular function.
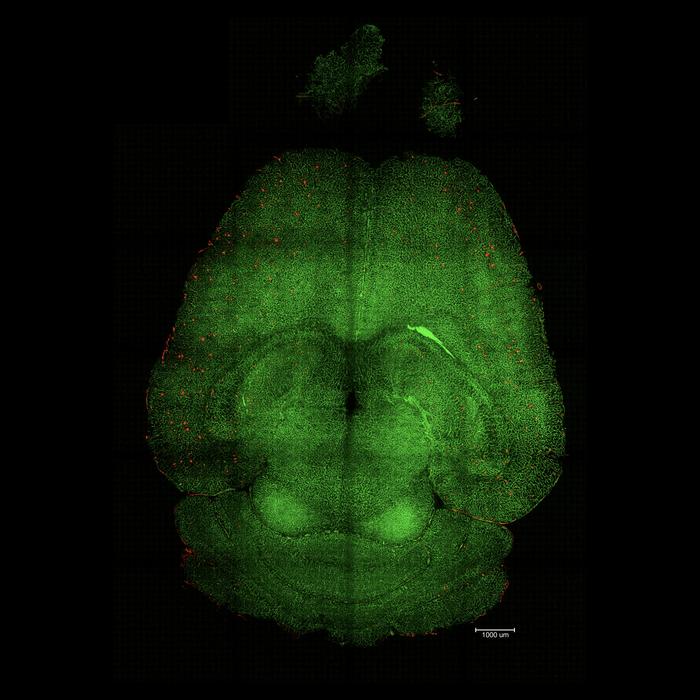

Brain After: Light sheet fluorescence microscope image of mouse brain 12h after being treated with nanoparticles. The brains were analysed to see the amount of Aβ plaques accumulation. Red: Aβ plaques. Green: vessels from the blood brain barrier. Credit: Institute for Bioengineering of Catalonia (IBEC)
A new therapeutic possibility
The nanoparticles are designed using a bottom-up molecular engineering approach, combining precise size control with defined surface ligands to interact with cellular receptors in a highly specific manner. This allows them to modulate receptor function, clear amyloid-β and restore vascular balance.
Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance
“Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance, restoring healthy function in the blood–brain barrier and leading to a striking reversal of Alzheimer’s pathology,” said Lorena Ruiz Perez, researcher at IBEC and Serra Hunter Assistant Professor at the University of Barcelona.
The study demonstrates how restoring the brain’s vascular function with bioactive nanoparticles can clear toxic proteins and reverse cognitive decline in mice. The findings could lead to further development pf new therapies that target vascular health to combat neurodegenerative diseases.
Related topics
Animal Models, Bioengineering, Central Nervous System (CNS), Drug Delivery, Drug Discovery, Nanomedicine, Nanotechnology, Neurosciences, Translational Science
Related conditions
Alzheimer's