How antibody-drug conjugates are improving gynaecological cancer outcomes
Posted: 13 November 2025 | Drug Target Review | No comments yet
Antibody-drug conjugates (ADCs) are emerging as a breakthrough in the fight against gynaecological cancers, offering targeted treatment for cervical, ovarian and uterine tumours.

Gynaecological cancers, particularly cervical, ovarian and uterine remain a serious threat to women’s health globally. Traditional treatments, including surgery and chemotherapy, can be effective, but many patients suffer from high recurrence rates, severe side effects and poor long-term outcomes. The search for more effective and targeted therapies has therefore become a key focus in oncology research.
One of the most promising developments in this area is the use of antibody-drug conjugates (ADCs), which have demonstrated potent anti-tumour activity and are reshaping treatment approaches for various solid tumours, including cervical and ovarian cancers. To date, two ADCs have received approval from the US Food and Drug Administration (FDA) for use in gynaecological cancers.
In a new review, researchers have outlined a detailed examination of the architecture, pharmacological mechanisms and molecular characteristics of approved and pipeline ADCs.
How ADCs work
ADCs are comprised of three key components: an antibody, a cytotoxic payload and a linker that connects them. The antibody is designed to specifically target tumour cells, delivering the toxic payload directly to the cancer. Once inside the cell, the payload is released, destroying the tumour from within while sparing healthy tissues. This targeted approach aims to maximise anti-cancer effects while minimising the collateral damage linked with conventional chemotherapy.
The accelerated FDA approval of the first ADC for gynaecological cancer, tisotumab vedotin, in 2021 for recurrent or metastatic cervical cancer, marked a significant milestone and spurred global research into ADC therapies.
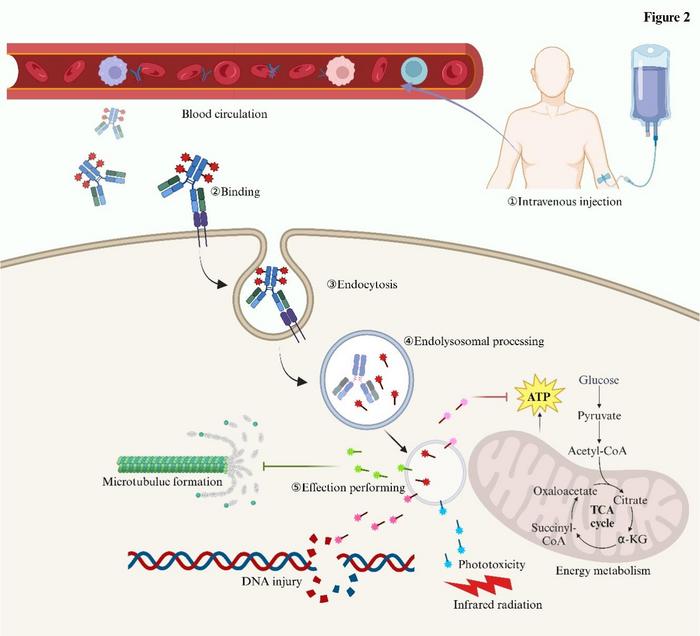

Following intravenous administration, ADCs circulate in the bloodstream (①) and specifically bind to their target antigen on the tumour cell surface (②). The antigen–ADC complex undergoes receptor-mediated endocytosis (③), after which the conjugate is trafficked into endolysosomal compartments, where proteolytic cleavage or linker degradation occurs to release the cytotoxic payload (④). The liberated payload exerts diverse effector functions (⑤), including inhibition of microtubule polymerisation, induction of DNA damage, disruption of energy metabolism, and in some cases phototoxicity upon irradiation, ultimately leading to tumour cell death. Credit: ©Science China Press
Expanding research and clinical trials
Since 2021, the development of ADCs has accelerated worldwide. Numerous ADCs are now in clinical trials, offering potential new treatment options for patients who have exhausted traditional therapies. Researchers are investigating a variety of tumour antigens as targets for ADCs, including FRα, HER2, TF, Trop2, mesothelin, B7-H4, CDH-6, NaPi2b and many others.
Since 2021, the development of ADCs has accelerated worldwide.
The ADCs under clinical and preclinical investigation offer new prospects for patients who have limited options with standard treatments. The growing pipeline of ADCs highlights the potential of these therapies to majorly improve outcomes for women with difficult-to-treat gynaecological cancers.
Managing side effects and looking ahead
While ADCs are highly targeted, they are not without side effects. Experts warn that these unique adverse effects require careful monitoring and management. However, the consensus in the field is optimistic: as research progresses, ADCs are expected to become more personalised and precise, improving their efficacy and safety.
As the study of gynaecological cancer treatment continues to evolve, ADCs could contribute to improved survival rates and a better quality of life for patients. With ongoing research and clinical innovation, the future may hold a new standard of care that is more effective and less invasive for people facing these cancers.
Related topics
Antibodies, Cancer research, Drug Delivery, Drug Development, Monoclonal Antibody, Oncology, Therapeutics
Related conditions
Cervical cancer, Ovarian cancer, uterine cancer