New genetically attenuated parasite gives hope for malaria vaccine
Posted: 17 June 2020 | Victoria Rees (Drug Target Review) | No comments yet
Using a genetically attenuated parasite (GAP) with a particular deleted gene, researchers have developed a new potential malaria vaccine that arrests at the late liver stage.


Scientists have reported that they have been able to develop a genetically attenuated parasite (GAP) that stops late in the liver stage of human malaria. According to the researchers, their findings pave the way for a novel, next generation vaccine against Plasmodium falciparum, the parasite that causes the deadliest form of malaria in humans.
The study was conducted by researchers at the Seattle Children’s Research Institute, US, who say a vaccine that targets the clinically silent phase of parasite infection in the liver and prevents blood stage infection, would not only decrease mortality in especially vulnerable groups like children under age five, but also block the cycle of transmission.
“Even the most promising experimental vaccine candidates, which can offer up to 52 percent protection in areas with intense malaria transmission, need to be improved upon to eliminate malaria,” said Dr Debashree Goswami, a fellow in the Kappe Lab at the research institute’s Center for Global Infectious Disease Research and lead author of the paper. “We must strive for 100 percent protection.”
To improve upon first generation GAPs, Goswami and her colleagues sought to engineer a GAP that develops in the liver stage for as long as possible before arresting.
To engineer their GAP, the researchers selected genes to delete from the parasite’s DNA, allowing the GAPs to stop in the liver without reaching the dangerous blood stage of infection. The arrest of these GAPs in the liver exposes them to the immune system and engenders a powerful and effective immune response that can block a new malaria infection from the moment a person receives an infectious mosquito bite.
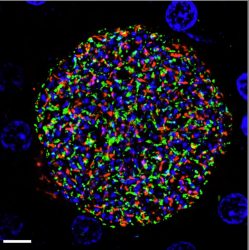

A Plasmodium falciparum liver stage parasite from the genetically attenuated parasite strain with the Mei2 gene deleted in a tissue section of an infected human liver-chimeric mouse liver. Antibodies against the parasite are labelled in red [credit: Seattle Children’s].
Laboratory studies in rodent malaria found late liver stage-arresting GAPs are far superior in generating robust immune responses when compared to early arresting GAPs. However, translating their results to the human malaria parasite proved challenging.
The researchers continued the search for a gene target that when deleted would allow the human malaria parasite to develop normally in the mosquito, undergo significant replication in liver cells and then arrest late in the liver stage. This led them to identify a gene in P. falciparum called Mei2.
“Deletion of Mei2 in both the rodent and human malaria strains caused the parasite to arrest very late in liver development,” Goswami said. “Importantly, we were still able to produce large numbers of sporozoites, the parasite stage that is at when transmitted from mosquito to human.”
The team then tested clones of P. falciparum with the Mei2 GAP in human liver-chimeric mice, a model used to study the human liver in the lab. They found that these GAPs replicated as expected and arrested very late during liver stage development and did not transition to the blood stage, like wild-type parasites without the genetic modification do.
“It’s promising to see the sustained prevention of transition to blood stage infection in the lab,” Goswami said. “For the first time, we have a viable P. falciparum late liver stage-arresting GAP we hope to advance for malaria vaccine development.”
Although Goswami cautions that the models used in their studies can’t fully mimic how the GAP might behave in an actual human liver, she believes there is sufficient evidence to move the Mei2 GAP vaccine candidate into early phase clinical trials.
The findings were published in JCI Insight.
Related topics
Immunology, Research & Development, Targets, Vaccine
Related conditions
Malaria
Related organisations
Seattle Children's Research Institute
Related people
Dr Debashree Goswami