Novel label-free assay developed to profile immune response to infection
Posted: 26 March 2021 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have created an immune assay that can profile host immune responses to infection and is faster than current methods.
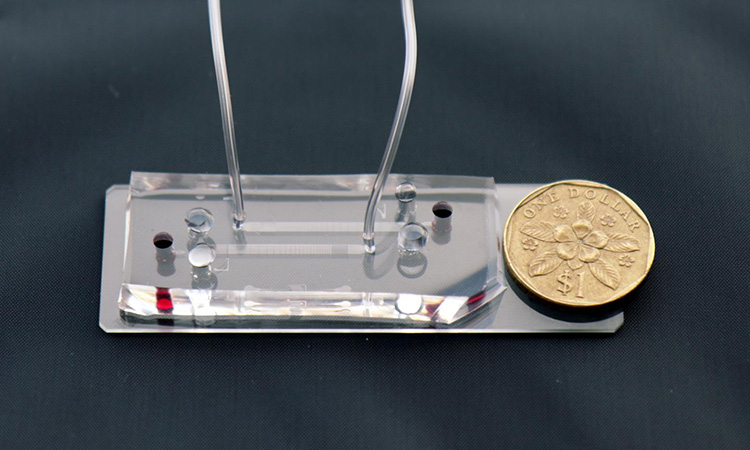
A closeup of the microfluidic DLD assay chip with the Singapore $1 coin for scale [credit: Singapore-MIT Alliance for Research and Technology (SMART)].
Researchers have developed a new label-free immune assay that profiles the rapidly changing host immune response in case of infection, in a departure from existing methods that focus on detecting the pathogens themselves, which can often be at low levels within a host. According to its developers, this technology presents a host of advantages over current methods, being both much faster, more sensitive and accurate.
The assay was developed by a team from Critical Analytics for Manufacturing Personalized-Medicine (CAMP), an Interdisciplinary Research Group (IRG) at the Singapore-MIT Alliance for Research and Technology (SMART), MIT’s research enterprise in Singapore.
The researchers say that in many cases, the main culprit behind disease manifestation, severity of infection and patient mortality is an overly aggressive host immune response. In cases of acute infection, the status of a patient’s immune response can often be volatile and may change within minutes. Hence, there exists a need for assays that are able to rapidly and accurately inform on the state of the immune system.
The new assay focuses on profiling the rapidly changing host inflammatory response, which in a hyper-aggressive state, can lead to sepsis and death. A 15-minute label-free immune profiling assay from 20μL of unprocessed blood using unconventional L and inverse-L shaped pillars of DLD microfluidic technology was developed, functioning as a sensitive and quantitative assay of immune cell biophysical signatures in relation to real-time activation levels of white blood cells (WBCs). As WBCs are activated by various internal or external triggers, the assay can sensitively measure both the extent and direction of these changes, which in turn reflect a patient’s current immune response state. As such, the assay is able to accurately and quickly assess patients’ immune response states by profiling immune cell size, deformability, distribution and cell counts.
The assay also provides considerable advantages over existing methods of profiling the immune system and its activity, the team say. These include measuring leukocyte gene expression, cell-surface biochemical markers and blood serum cytokine profile. Notably, these current methods require sample dilution or pre-processing steps, as well as labour-intensive, expensive equipment and antibody labelling procedures. As a result, these methods generally require a few hours, at minimum, to return results.
In contrast, the new assay takes only 15 minutes, uses only 20μL of whole blood and only requires video capture frame rates of up to 150fps, there is considerable potential for the technology to be developed into a portable unit that can perform point-of-care blood-sparing assays which could significantly improve the diagnosis and differentiation of patients. This application will enable clinicians to be able to quickly identify at-risk patients and take immediate action to mitigate or prevent organ dysfunction and other adverse effects of a hyper-aggressive immune response.
“Our new deterministic lateral displacement (DLD) assay will help address an unmet need in the ER and intensive care unit (ICU) by significantly reducing waiting time for accurate patient assay results. This could lead to more effective triage decision-making and more appropriate and timely treatment, which are critical to saving lives. More generally, this ground-breaking technology provides new insights into both the engineering of precision microfluidics and clinical research,” said lead author Dr Kerwin Kwek Zeming.
Professor Jongyoon Han, co-author on the paper, added: “In the wake of lessons learnt in emergency rooms in hospitals across the world especially during the COVID-19 pandemic, where medical professionals have been faced with making difficult and at times life-or-death decisions in triage, this new technology represents a hugely exciting and significant breakthrough. By reducing the time taken for assay results from hours to a matter of minutes, SMART CAMP’s new assay could help save lives as we continue to combat the scourge of pathogens and infectious diseases. The assay will also have wider applications, giving clinicians a new and more effective tool in the ER and ICU.”
The study was published in Small.
Related topics
Assays, Immunology, Microfluidic Technology
Related organisations
Singapore-MIT Alliance for Research and Technology (SMART)
Related people
Dr Kerwin Kwek Zeming, Professor Jongyoon Han