Novel antibody could reduce SARS-CoV-2 transmission
Posted: 11 February 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Researchers at UC Davis Health have engineered an antibody that interferes with a critical cell mechanism to reduce transmission of the virus that causes COVID-19 in lab tests.

A novel antibody named FuG1, developed at the University of California (UC) Davis Health, US, targets the enzyme furin, which viruses use to create an efficient chain of infection in human cells. According to the researchers, this approach could be added to the existing SARS-CoV-2 antibody fusion to work against emerging variants. Engineered FuG1 antibody also competitively interferes with the furin function needed for SARS-CoV-2 to become highly transmissible. The study which evaluates the efficiency of the engineered antibody has been published in Microbiology Spectrum.
“We developed an approach that interferes with the transmission chain of SARS-CoV-2. The COVID-19 vaccines are a great lifesaver in reducing hospitalisations and severe illness. Yet, we are now learning that they may not be as effective in controlling the transmissibility of the virus,” said Jogender Tushir-Singh, senior author of the study.
Furin, found throughout the human body, is involved in various functions of cells. It is a type of enzyme, a protease, that can break down proteins into smaller components. It does so by cleaving the polybasic peptide bonds within proteins. In cleaving these bonds, furin often acts as a switch, changing an inactive protein into an active one. It can also cleave and activate viruses that enter human cells. When SARS-CoV-2 infects a human cell, it is in its active state, having already “cleaved” its spike protein, a key protein that binds to ACE2 receptors to gain entry. However, when the virus is being synthesised within the host cell, the spike is in an inactive state. The virus needs to use the host cell’s furin to cut the spike protein into two parts, S1 and S2, which makes the spike active on the viral particles for efficient transmissibility upon release.
Reduce preclinical failures with smarter off-target profiling
24 September 2025 | 15:00PM BST | FREE Webinar
Join this webinar to hear from Dr Emilie Desfosses as she shares insights into how in vitro and in silico methods can support more informed, human-relevant safety decisions -especially as ethical and regulatory changes continue to reshape preclinical research.
What you’ll learn:
- Approaches for prioritizing follow-up studies and refining risk mitigation strategies
- How to interpret hit profiles from binding and functional assays
- Strategies for identifying organ systems at risk based on target activity modulation
- How to use visualization tools to assess safety margins and compare compound profiles
Register Now – It’s Free!
However, the researchers highlight that inhibiting furin to limit the SARS-CoV-2 chain of infection cycle is not a simple mechanism. Furin is found throughout the human body and is vital for many biological processes. Stopping furin causes high body toxicity, which is why standard furin-inhibiting drugs are not clinically feasible. Instead, the team created a new approach by engineering a conjugated antibody targeting the SARS-CoV-2 spike protein. The design is similar to therapeutic monoclonal (IgG) antibodies but includes an added feature — Fc-extended peptide — that specifically interferes with the host furin.
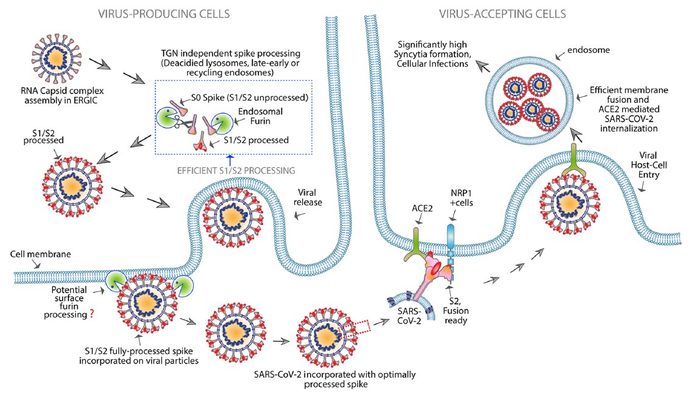

Engineered FuG1 antibody competitively interferes with the furin function needed for the SARS-CoV-2 virus to become highly transmissible [credit: UC Regents].
FuG1 allows the interruptions of the furin function to limit spike activation, thus specifically limiting the viral transmissibility during its chain of infection in host cells. The high affinity, variable-domain-targeting spike in FuG1 is key for furin-targeting specificity to avoid potential toxicity.
The team evaluated the engineered furin disrupter, FuG1, in human lung tissue cells. Tests were run with the original SARS-CoV-2 variant and pseudoviruses. They found that:
- Adding the furin disruptor peptide did not interfere with the function of the antibody or its ability to bind to the SARS-CoV-2 spike
- FuG1 significantly impacted the spike cleavage at furin sites
- FuG1 additionally interfered with the overall stability of the SARS-CoV-2 spike protein, which in general is necessary for infecting cells and transmissibility of the virus.
The next steps for the team will be a series of experiments in mice. They will also test the engineered antibody against current variants like Omicron.
In addition to targeting SARS-CoV-2, Tushir-Singh thinks this approach could be applied to future coronaviruses or any other virus that utilises proteases like furin to infect cells for their pathology.
Related topics
Antibodies, Antibody Discovery, Drug Leads, Protein, Therapeutics
Related conditions
Coronavirus, Covid-19
Related organisations
University of California (UC) Davis Health
Related people
Dr Jogender Tushir-Singh