Identifying radioresistance drivers in glioblastoma multiforme
Posted: 1 October 2020 | Hannah Balfour (Drug Target Review) | No comments yet
According to researchers, Rab27b and epiregulin contribute to the development of radioresistance and could be targeted to improve glioblastoma patient survival.
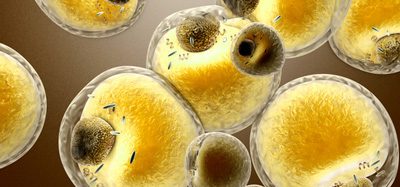
![Glioblastoma Multiforme (GBM) cells in culture [Credit: Dr Jin-Min Nam].](https://www.drugtargetreview.com/wp-content/uploads/glioblastoma-cells-750x500.jpg)
![Glioblastoma Multiforme (GBM) cells in culture [Credit: Dr Jin-Min Nam].](https://www.drugtargetreview.com/wp-content/uploads/glioblastoma-cells-750x500.jpg)
Glioblastoma Multiforme (GBM) cells in culture [Credit: Dr Jin-Min Nam].
An international collaboration has identified two key molecules that mediate radioresistance (resistance to radiotherapy) in glioblastoma multiforme (GBM). The findings suggest these molecules, Rab27b and epiregulin, are potential targets for the treatment of this brain cancer.
GBM is the most aggressive type of brain cancer. While it is currently treated using a combination of radiation therapy and chemotherapy, the ability of the cancer to rapidly develop radioresistance via unknown mechanisms means the five-year survival rate is less than seven percent.
Understanding how radioresistance develops in GBM is the focus of research by a team of scientists from Hokkaido University, Japan, and Stanford University, US. In their study published in Neuro-Oncology Advances, they explained how two key molecules, Rab27b and epiregulin, interact to contribute to radioresistance in GBM.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
According to the researchers, they focused on Rab27b because it is known to promote tumour progression and metastasis in several types of cancer. Its primary function is to regulate protein trafficking and secretion of molecules.
The team used human GBM cell lines to demonstrate that Rab27b expression was increased for at least seven days after exposure to radiation. In the study, knockdown of Rab27b increased the sensitivity of glioblastoma cells to irradiation.
These tests were also replicated in an animal model, where the glioblastoma cells were injected into mice prior to them being subjected to radiation therapy. The scientists report that Rab27b knockdown combined with radiation therapy delayed tumour growth and prolonged mouse survival time.
![Tumour growth in mice injected with the glioblastoma cells. After radiotherapy, the growth of tumour with normal Rab27b expression (top row) is much more rapid than that of the tumour with knocked down Rab27b (bottom row) [Credit: Soichiro Nishioka, et al., Neuro-Oncology Advances, August 8, 2020].](https://www.drugtargetreview.com/wp-content/uploads/Glioblastoma-Rab27b-knockout-diagram-750x207.jpg)
![Tumour growth in mice injected with the glioblastoma cells. After radiotherapy, the growth of tumour with normal Rab27b expression (top row) is much more rapid than that of the tumour with knocked down Rab27b (bottom row) [Credit: Soichiro Nishioka, et al., Neuro-Oncology Advances, August 8, 2020].](https://www.drugtargetreview.com/wp-content/uploads/Glioblastoma-Rab27b-knockout-diagram-750x207.jpg)
Tumour growth in mice injected with the glioblastoma cells. After radiotherapy, the growth of tumour with normal Rab27b expression (top row) is much more rapid than that of the tumour with knocked down Rab27b (bottom row) [Credit: Soichiro Nishioka, et al., Neuro-Oncology Advances, August 8, 2020].
As Rab27b is a regulator of protein trafficking, the scientists continued looking for other molecules that could contribute to radioresistance. They discovered that changes in the expression of Rab27b led to corresponding changes in the expression of epiregulin, a growth factor whose expression is known to increase in cancer cells. The team experimented with knocking down the expression of epiregulin and found it increased the sensitivity of GBM to irradiation, as seen in the cells with Rab27b knockdown.
The scientists also confirmed that increased expression of Rab27b and epiregulin in GBM induced the proliferation of surrounding cancer cells, which could contribute to the acquisition of radioresistance. In a final test, they analysed gene expression data of GBM patients and found that upregulation of Rab27b and epiregulin correlated with poor prognosis.
According to the researchers, their study indicates Rab27b and epiregulin play a role in the development of radioresistance in GBM and are therefore novel targets for drug development.
Dr Jin-Min Nam and Dr Yasuhito Onodera are part of the Radiation Biology group at the Global Center for Biomedical Science and Engineering (GCB), a collaboration between Hokkaido University and Stanford University. The group specialises in molecular and cellular oncology and radiation biology.
Related topics
Disease Research, Drug Targets, In Vitro, In Vivo, Oncology
Related conditions
Glioblastoma multiforme
Related organisations
Hokkaido University, Stanford University
Related people
Dr Jin-Min Nam, Dr Yasuhito Onodera