Algae: a source for prebiotics and drugs to treat IBD
Posted: 23 May 2023 | Dr Dorit Avni, Dr Federica Ungaro, Dr Maria Hayes, Professor Kiron Viswanath | No comments yet
An increasing number of people worldwide suffer from inflammatory bowel disease (IBD). However, no treatment is effective for all patients. In this article, researchers working on the Algae4IBD project explain how algae may represent a valuable source of prebiotics and new therapeutic agents for IBD and other diseases.

Inflammatory bowel disease (IBD) is an umbrella term that describes complex disorders that cause chronic inflammation in the digestive tract with alternating periods of relapse and remission. More than 6.8 million people are diagnosed with IBD, which can occur in adults, adolescents and children under the age of 10 and whose quality of life is substantially affected by it.
Available interventions are also costly, with healthcare costs to treat the condition averaging more than €5,000 per patient each year in Europe.
Therapies developed in recent decades have transformed the treatment of IBD, making hospitalisation and surgery less common. However, many patients respond poorly to corticosteroid treatment, or their immune system responds unfavourably to biological therapies, such as the development of autoimmune diseases. Furthermore, available interventions are also costly, with healthcare costs to treat the condition averaging more than €5,000 per patient each year in Europe.1 The growing economic burden of IBD is due to its rising prevalence combined with therapeutic costs, necessitating new cost-effective treatments and algae represent a promising source.
Prebiotics from algae to treat IBD
IBD arises from an interaction between genetics and environmental factors such as diet and lifestyle. In addition, the composition of the gut microbiota in IBD patients is reshaped to favour a group of bacteria that cause inflammation in the gastrointestinal tract such as Escherichia coli (E. coli), Clostridium difficile, Ruminococcus and Klebsiella, which are suspected to be involved in the development of IBD (Figure 1).
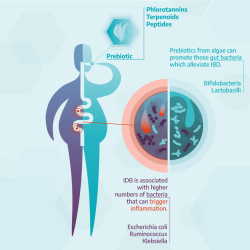

A changed composition of the gut microbiota is a characteristic trait of inflammatory bowel disease. Algae-based compounds with prebiotic effects have the potential to restore a healthy microbiome composition and alleviate disease symptoms.
Credit: European Science Communication Institute
The human microbiota is fundamental to correct functioning of tissue homeostasis, actively interacting with the host on different levels including mucosal surfaces of which the gastrointestinal tract is the most colonised. For instance, Ruminococcus gnavus produces mucolytic agents and can degrade the protective mucus lining of the digestive organs. This allows pathogenic microorganisms such as E. coli to access the epithelium. Oral bacteria belonging to the genus Klebsiella are known to have the capacity to colonise the intestine and proliferate T helper 1 cells,2 immune cells that fight off pathogens, but are also implicated in autoimmune reactions.3 Additionally, the increase in pathogenic microbe levels could supplant the ‘good’ microorganisms like bifidobacterial and lactobacilli that have been shown to alleviate symptoms of IBD.
A healthy physiological condition requires all microbiota species to coexist in a continuous and dynamic equilibrium. Diet plays a crucial role in the composition of the gut microbiome and understanding the complex interplay between diet, host microbiome and host health is an ongoing endeavour. For example, it has been shown that a certain intake of animal protein can increase the abundance of lactobacilli.4
Diet can not only change the numbers of microorganisms in the gut, but also influence the metabolite pool in the intestine. For instance, microbial fermentation products such as branched-chain fatty acids can increase inflammation and contribute to the development of IBD,5,6 while bacterial fermentation products such as short-chain fatty acids are known to benefit gut health by restoring the gut microbiota’s natural state. Hence, we believe that prebiotics that promote the proliferation of beneficial bacteria and the production of health-beneficial short-chain fatty acids can not only prevent the onset of IBD, but also alleviate its symptoms.5
Algae represent a promising source for prebiotic development.
Several polysaccharides from the green seaweed Enteromorpha clathrata promote the growth of health-beneficial bacteria such as bifidobacterial and lactobacilli and polysaccharides from the microalga Chlamydomonas reinhardtii possess antibacterial and antibiofilm activity against pathogens like Klebsiella pneumoniae. The algae-derived non-digestible oligosaccharides can also influence the expression of cytokines that regulate the immune system. Fucoidan from the brown seaweed Cladosiphon okamuranus and an extract from the red seaweed Eucheuma cottonii were found to reduce proinflammatory cytokines while increasing anti-inflammatory cytokines.7 Thus, algal oligosaccharides and polysaccharides with potential use as prebiotics could reduce inflammation in the intestine of IBD patients.5
Such algal compounds could be formulated for patients to take orally or to include in dairy products, as their fat content preserves the algae prebiotic until it reaches the intestine. In the form of enriched yoghurts, the benefits of prebiotics from algae offer access to a broader consumer group. Both capsules and functional food can additionally contain probiotic bacteria for a symbiotic approach, leveraging the synergistic effects of prebiotics and probiotics.
Algae – an overlooked resource for new therapies
It is only in recent years that algae research has expanded beyond their potential for producing biodiesel, to also consider them as a bio-resource, not only for novel prebiotics but also for compounds with unique structures and pharmaceutically relevant bioactivities. Micro- and macroalgae from both marine and fresh water produce small molecules and secondary metabolites with aggregative, anti-inflammatory, antioxidant, antimicrobial, anti-viral and anti-tumoural bioactivities. These include carotenoids like astaxanthin, polyunsaturated fatty acids such as docosahexaenoic acid, or phenolic compounds, but also other compounds that have so far only been found in algae. Examples include phlorotannins from brown seaweeds and small molecules like terpenoids and peptides, found in microalgae.
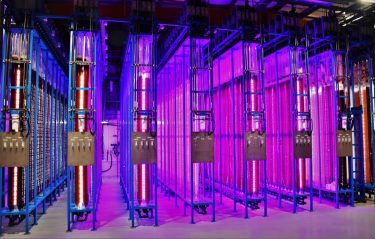

With new photo-bioreactors and advanced monitoring of cultures, microalgae can be cultivated under controlled conditions. Outdoor cultivation seizes natural sunlight (left), while the light conditions could be controlled for indoor photo-bioreactors (right).
Credit: Necton and Yemoja Ltd.
Natural product research is as relevant as ever, and its success rate benefits from recent methodological developments in high-throughput in vitro screenings, single-cell and multi- cell omics for compound detection.8 Advances in mass-spectrometry and NMR technologies, pre-clinical testing using human ex vivo and in vivo models, synthetic biology and biorefinery processes, can improve success in characterising, testing and producing identified compounds. Cultivation methods for microalgae continue to improve (Figure 2), as does our understanding of how production of a target compound can be triggered by changing culture conditions. For instance, the expression level of anti-inflammatory compounds often depends on nutrient starvation, extreme temperature and light intensity.9


Academic scientists researching natural products often struggle with the pitfalls of drug development. To overcome this, research groups should foster collaborations with peers across all fields and with pharma companies to replicate the entire production chain: identification and screening of sources, isolation and characterisation of compounds, preclinical assessment through various approaches (in vitro, ex vivo human cell models and mice models), production and application of the target compounds, and critical manufacturing controls. Pharmaceutical companies meanwhile need to reinvest in natural product research. Since methodologies such as combinatorial chemistry have not had the anticipated success rates, drug discovery now depends on new, natural compounds.
All in all, research into natural compounds from algae becomes increasingly feasible and can lead to the discovery of many novel and unique prebiotics, bioactive compounds and drugs. Their combined action is needed to treat complex diseases such as IBD.
Multifaceted treatment for a multifaceted chronic disease
Successful IBD treatment prolongs remission periods, improves the patients’ quality of life and reduces hospitalisation. Achieving these improved care outcomes requires a holistic approach that considers all aspects of this complex disease such as the patient’s status and the composition of the gut microbiome.
Although results are not consistent, different microbial compositions may be associated with IBD onset, progression (remission/relapse), and the treatment regimens that patients undergo. For example, higher numbers of Ruminococcus were observed at the onset of IBD and the number of bifidobacteria is reduced during flare-ups of Crohn’s disease, a form of IBD. In general, the more severe IBD is, the less abundant bifidobacteria are.10 Knowing more about these changes in the intestinal environment is key to better understand IBD pathogenesis and develop successful interventions to target the microbiota effectively.
Future therapies must be oriented towards the gut microbiome, because standard treatment regimens that only administer drugs may not be enough to get control over IBD. The much-needed alternative approaches to treat this disease should take a two-step approach: using prebiotics and drugs in synergy to counteract symptoms such as inflammation while re-establishing a healthy gut microbiome. Such therapies should acknowledge and respond to the distinct features of the different stages of IBD and their respective microbiota composition. Due to their antioxidative, anti-inflammatory, and enzyme inhibitory activities and their antibacterial and growth promoting potential, active compounds from algae may act as prebiotics and therapeutic compounds. Exploring this unexploited resource through revived natural product research represents a new avenue for pharma companies to take on the challenges of IBD and other diseases.
Limitations when commercialising algae or their compounds
Identifying multiple, beneficial algal bio actives has the potential to benefit the lives of IBD sufferers, yet several hurdles must first be overcome. Contamination control during microalgae cultivation is key as the quality of microalgae cultures can affect the quality of the functional food or pharmaceutical product. Undesirable microbial growth can occur in traditional production processes such as raceway pond systems. These are ‘open pools’ in which a paddle wheel circulates the algae cells and nutrients. They facilitate cheaper production costs but are more prone to contamination. Controlled production of microalgae in bioreactors will help to overcome this issue. However, high value bio actives must be identified to make bioreactor production economically feasible. In addition, bioreactor operation must become cheaper and algae producers are already developing innovative production approaches.
Another caveat for feasible use of microalgae concerns the production of functional foods. To enrich food with algae-based prebiotics you must use an approved algae permitted for use as a novel food or functional food ingredient in the EU, from a list established by the European Food Safety Authority (EFSA). The legislation governing this list is for consumer protection and also applies to algae that are intended for use as food. Regulation (EU) 2017/2470 maintains this list within the novel food catalogue. It contains all authorised novel foods permitted for sale within the EU. The Novel Food Catalogue includes both European and imported algae, of which there are currently 22.11 Only six microalgae are approved for use as novel food in the EU: Arthrospira platensis, Chlorella luteoviridis, Chlorella pyrenoidosa, Chlorella vulgaris, Chlamydomonas reinhardtii and Spirulina sp.. Approval requires meeting all necessary safety, nutritional and bioactivity requirements of EFSA, but microalgae and algae in general hold enormous potential. This potential should be seized to bring the prebiotic and health promoting benefits of algae to people who need it.


Dorit leads the Sphingolipods, Phytochemicals and Immune Modulation Lab at the MIGAL Galilee Research Institute. Her lab applies bio-active metabolites as immune system regulators to prevent and treat diseases, and develops novel platforms to treat unmet diseases such as IBD. Dorit also coordinates the EU-funded research project Algae4IBD. There she leads an interdisciplinary research team that replicates the entire drug development and functional food ingredient pipeline to combat inflammation, pain and IBD including cell-based and preclinical models of IBD.


Professor Kiron Viswanath
Kiron is Leader of the Algal and Microbial Biotechnology Division in the Faculty of Biosciences and Aquaculture at the Nord University, Norway. He currently examines the impact of algal compounds on the microbiome in the Algae4IBD project. He employs in vitro and in vivo approaches to understand the proliferative (prebiotic-induced) and inhibitory (antimicrobial) effects of polysaccharides/polyphenols on the intestine bacterial community.


Dr Maria Hayes
Maria is Senior Scientific Researcher at Teagasc, the Irish Agricultural and Food Development Authority. Her research interests include the isolation and characterisation of new natural products. In the Algae4IBD project, Maria is responsible for the generation and identification of novel prebiotics and cyclooxygenase inhibitors from macro and microalgae and functional food product development with identified, potential lead extracts and compounds.


Dr Federica Ungaro
Federica is head of the Experimental Gastroenterology Laboratory at San Raffaele Hospital, Italy. Her current lines of research exploit NGS and omics science to unveil and demonstrate the mechanisms underlying intestinal inflammation pathogenesis. Moreover, she has been investigating the role of the gut microbiota in IBD and how it can be modulated. In the Algae4IBD project she will investigate the impact of algae-based compounds on intestinal physiology and microbiota in both human and murine preclinical models of IBD.
References:
- Linschoten et al. (2021) Systematic review: societal cost of illness of inflammatory bowel disease is increasing due to biologics and varies between continents, Aliment Pharmacol Ther., 54:234 – 248, https://doi.org/10.1111/apt.16445
- Atarashi, et al. (2017) Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation, Science, 358 (6361), 359-365, https://www.science.org/doi/full/10.1126/science.aan4526
- Skapenko, et al. (2005) The role of the T cell in autoimmune inflammation, Arthritis Res. Ther., 7 (Suppl 2), S4 (2005). https://doi.org/10.1186/ar1703
- Zhu et al. (2016) Intake of meat proteins substantially increased the relative abundance of genus lactobacillus in rat feeces, Plos One, 11 (4), e0152678, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4820228/
- Kiron et al. (2022) Inflammatory bowel disease – A peek into the bacterial community shift and algae-based „biotic“ approach to combat the disease, Trends Food Sci Tech, 129, 210 – 220, https://doi.org/10.1016/j.tifs.2022.09.012
- Diether & Willing (2019) Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction, Microorganisms, 7(1), 19, 10.3390/microorganisms7010019
- Besednova et al. (2020) Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine, Mar Drugs., 18(6), 289, 10.3390/md18060289
- Zhu et al. (2022) New opportunities and challenges of natural products research: When target identification meets single-cell multiomics, Acta Parhm Sin B, 12 (11), 4011 – 4039, https://doi.org/10.1016/j.apsb.2022.08.022
- Montero-Lobato et al. (2018) Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae, Marc Drugs, 16 (12), 478, https://doi.org/10.3390/md16120478
- Aldars-Garía et al. (2021) Systematic review: The gut microbiome and its potential clinical application in inflammatory bowel disease, Microorganisms. 9 (5), 933, https://doi.org/10.3390/microorganisms9050977
- Novel Food Catalogue [Internet]. food.ec.europa.eu. Available from: https://food.ec.europa.eu/safety/novel-food/novel-food-catalogue_en
Related topics
Bacteriophages, Biopharmaceuticals, Drug Delivery, Drug Development, Drug Discovery, Drug Discovery Processes, Microscopy, Stem Cells, Targets
Related conditions
Inflammatory bowel disease (IBD)
Related organisations
Algal and Microbial Biotechnology Division, Irish Agricultural and Food Development Authority, MIGAL Galilee Research Institute, San Raffaele Hospital
Related people
Dr Dorit Avni, Dr Federica Ungaro, Dr Maria Hayes, Professor Kiron Viswanath








