Cancer drug discovery: protein-ligand interactions
Posted: 1 November 2023 | Taylor Mixides (Drug Target Review) | No comments yet
Dr Rob van Montfort, a leading researcher at the Centre for Cancer Drug Discovery (CCDD) within The Institute of Cancer Research, London, is at the forefront of cancer drug discovery. Focusing on protein-ligand interactions, his work utilises structural biology and biochemical methods to help the discovery of novel small-molecule drugs for the treatment of cancer. At CCDD, a unique academic drug discovery centre with an industry-like approach, Dr van Montfort leads the Hit Discovery and Structural Design Team. This multidisciplinary team plays a pivotal role in characterising protein-ligand interactions, employing an array of methods, from high-throughput screening to biophysical techniques like Surface Plasmon Resonance (SPR) and structural methods such as X-ray crystallography and cryo-electron microscopy. Their mission is to guide the optimisation of initial screening hits towards potent inhibitors with the potential for clinical impact.

Could you provide an overview of your research on target directed cancer drug discovery, particularly your focus on protein lagging interactions. How do structural biology and biochemical methods play a role in your world?
I work in the Centre for Cancer Drug Discovery (CCDD) at The Institute of Cancer Research in London, which is an academic drug discovery centre. The mission of the CCDD is to discover novel small-molecule therapeutics for the treatment of cancer and progress them to hypothesis testing phase 1 clinical trials. Although we are an academic centre we work very much like industry. The team that I lead within the CCDD is called the Hit Discovery and Structural Design Team. We support the drug discovery projects of the CCDD with assay development and screening, biophysics, structural biology and recombinant protein production. With all these diverse methods we characterise protein-ligand interactions in many ways. For example, in the screening stage of a project we are trying to find initial starting points for optimisation by our medicinal and computational chemistry colleagues.
In terms of compound libraries, we have a high-throughput screening library of about 200,000 ligands. For fragment screening, which are much smaller compounds, we have a library of about 3000 fragments. We can screen with many different formats and modalities. We often screen using a biochemical assay, but we can conduct cell-based screening as well. For fragment screening, we can screen with biophysical methods, such as thermal shift assays or, you may have heard about Surface Plasmon Resonance (SPR) which is a very sensitive biophysical technique used for fragment screening. We can look at the detailed binding modes of our compounds bound to their protein targets in X-ray crystallography, and for drug targets or complexes that are difficult to crystallise we can use cryo-electron microscopy (cryoEM) as well. So that is a wide range of techniques to look at the protein-ligand interactions, all with the aim to guide the optimisation of our first screening hits to potent inhibitors that hopefully are cell-active at some point.
Can you discuss the transition from industry to academia and how it’s influenced your approach to cancer drug discovery?
Moving from industry to academia, to the Centre for Cancer Drug Discovery at the ICR that I work in now was not that difficult because although we work within the academic environment we operate as a biotech company. At CCDD we have a lot of capabilities and skills to do modern drug discovery including biologists, assay scientists, biochemists, biophysicists, computational chemists, medicinal chemists, computational biologists, structural biologists, DMPK and in vivo scientists. We are organised in teams, but work in multidisciplinary project teams, which is the type of research environment that you will find in industry.
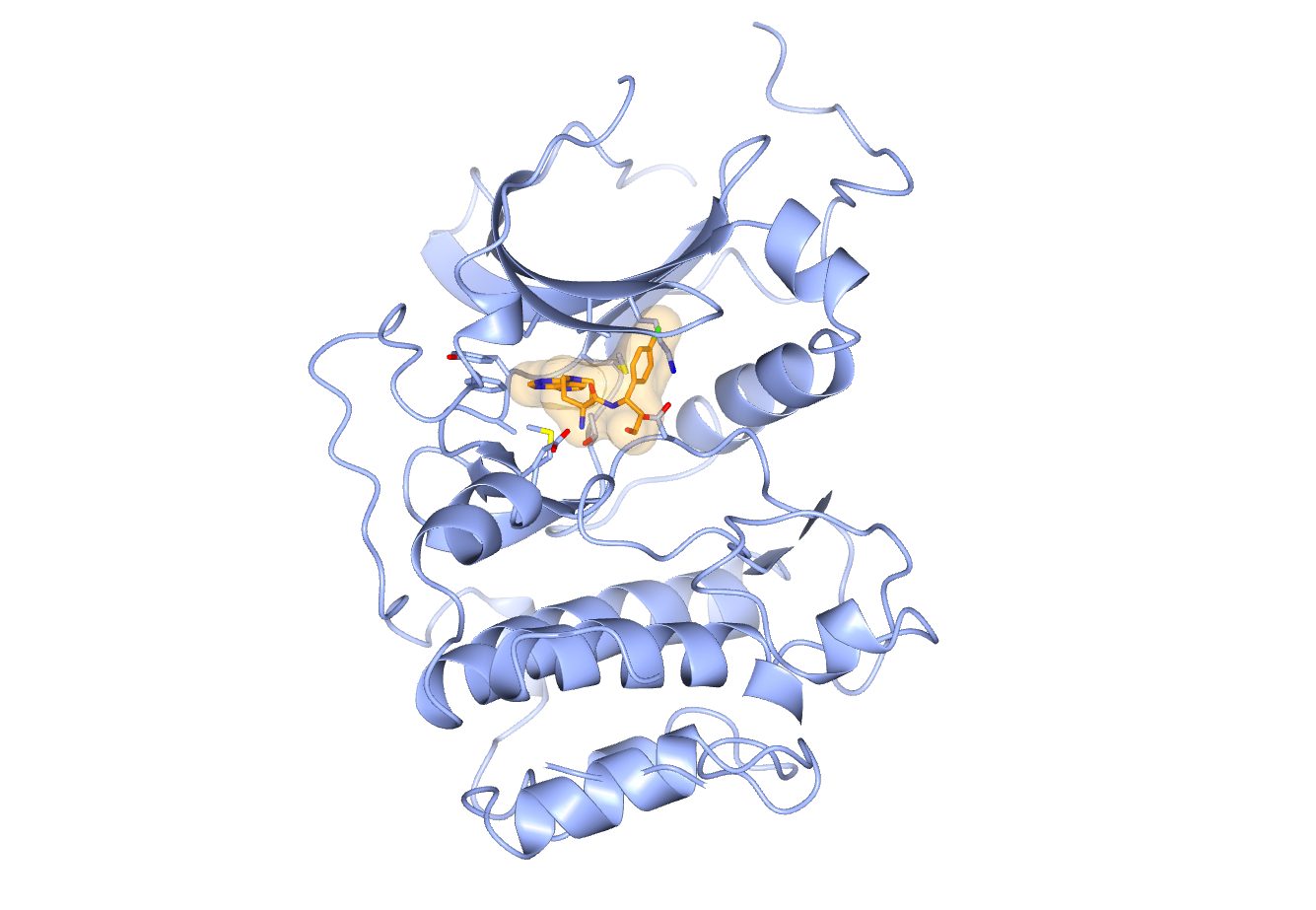
Capivasertib (orange) bound to the kinase domain of AKT (blue). The figure was generated with CCP4MG (McNicholas et al. Acta Cryst. (2011) D67, 386-394) using PDB ID 4GV1 which was published as part of Addie et al. J. Med. Chem. (2013) 56, 2059-2073.
What is different though, is that because we are an academic drug discovery centre, publication of our drug discovery research is integral to our mission. In addition, and in line with ICR’s strategy, part of our remit is to train the next generation of cancer drug discoverers. We are part of the ICR’s PhD-student programme, which is exciting, because with PhD-student projects we have the ability to probe the more academic questions related to our drug discovery science. Our PhD-studentships also allow for exciting collaborations with other scientists at ICR and externally, including collaborative PhD-projects with industry.
Another key difference with industry is the close partnership with the Royal Marsden Hospital, the first hospital in the world dedicated to cancer diagnosis, treatment, research and education. Both of the ICR’s research laboratory sites in Sutton and in Chelsea are in close proximity to the Royal Marsden Hospital sites. There are many joint appointments between ICR and Royal Marsden Hospital including some the group leaders in the CCDD who are clinicians in the Royal Mardsen Hospital. This arrangement enables comprehensive and timely feedback from the clinic into our drug discovery research, so it is a very important interaction for us. On a personal level, the co-location of the ICR and the Royal Marsden Hospital has a significant impact as well. You only have to walk through one corridor to get from the ICR to the Royal Marsden and go from your own daily research to being exposed to the patients and staff in the hospital. That is quite a powerful reminder and driver why we are doing our drug discovery research.
Your work involves the use of various assays, X-ray crystallography, sorry if I said that wrong, cryo electron microscopy for hit discovery and inhibitor design. Can you elaborate on how these techniques are integrated into your research process and how they contribute to advancing cancer drug discovery?
We are involved from a very early stage once a target is validated. We need to build the assays that we need for compound screening. For biochemical assays, biophysics, and the structural techniques, we will need to generate recombinant versions of the protein drug target. Often the different methods require different variants of the protein target, so that can be quite a lot of work. We stay involved with all our methods and approaches throughout the lifetime of a project, first to help optimise the potency of the inhibitors and later to help assess if our inhibitors inhibit their target in a cellular context. With a structural technique such as X-ray crystallography we provide pictures of our inhibitors bound to their protein targets to help inform our computational and medicinal chemists in the design of improved inhibitors which is an iterative and very creative process.
In your role as the leader of the Hit Discovery and Structural Design Group, what are some recent or ongoing projects that you find particularly exciting or promising future?
We have several exciting projects in various stages of discovery, but a small-molecule drug that is causing a lot of excitement at the moment is Capivasertib. Capivasertib originated from a collaboration between my former company Astex Pharmaceuticals and the ICR and was further developed by AstraZeneca. Capivasertib inhibits a signalling protein called AKT and recently gave very promising results in the phase three clinical trial testing its effectiveness in combination with another drug called Faslodex for the treatment of patients with hormone driven metastatic breast cancer. Hopefully Capivasertib will get approved as a drug at some point and that is why all of us are watching this space very closely.
What advice would you give to aspiring researchers looking to make a significant impact in cancer drug discovery?
I think as a researcher you have to be honest and transparent about your data, and always do the best science that you possibly can do. You need to be resilient because science is difficult and things will fail. Therefore, it is very important not to forget to celebrate your successes. In terms of a career, if you are passionate about science, whether that is cancer research or another field, you have to follow your dreams.
About the author
 Dr Rob van Montfort
Dr Rob van Montfort
Reader in Structural Biology and Cancer Drug Discovery
Dr Rob van Montfort studies target-directed cancer drug discovery, focusing on protein-ligand interactions, using structural biology and a range of biochemical and biophysical methods.
He received his undergraduate degree in Chemistry and PhD in structural biology at the University of Groningen in the Netherlands. He did his postdoctoral research at Birkbeck College (University of London, UK), where he became interested in proteins involved in stress response. In 2001, he joined Astex Pharmaceuticals in Cambridge where he became an expert in fragment-based drug discovery working on diabetes, thrombotic and oncology drug targets.
In 2007 Dr van Montfort joined the Institute of Cancer Research (London, UK), where he holds a joint position between the Centre for Cancer Drug Discovery (CCDD) and the Division of Structural Biology. Since 2010 he leads the Hit Discovery and Structural Design group, which uses biochemical, cellular and biophysical assays to perform small-molecule high-throughput screening and fragment-based hit discovery, coupled with X-ray crystallography and cryo-electron microscopy (cryoEM) to guide inhibitor design for the drug discovery project in the CCDD portfolio. He closely collaborates medicinal and computational chemists, biologists and DMPK experts at the ICR and externally. He is a named recipient of the Team Science Award 2012 from the American Association for Cancer Research (AACR) which was awarded to the Cancer Research UK Cancer Therapeutics Unit and Drug Development Units of the Institute of Cancer Research (ICR) and Royal Marsden Hospital. In 2019 he became Reader in Structural Biology and Cancer Drug Discovery.
Related topics
Drug Discovery, Drug Discovery Processes, Immunotherapy
Related conditions
Cancer
Related organisations
Centre for Cancer Drug Discovery
Related people
Dr Rob van Montfort (Centre for Cancer Drug Discovery)