The future of cancer immunotherapy with Elicio Therapeutics
Posted: 1 March 2024 | Dr Christopher Haqq (Elicio), Taylor Mixides (Drug Target Review) | No comments yet
In this exclusive interview with Dr Christopher Haqq, Executive Vice President, Head of Research and Development, and Chief Medical Officer of Elicio Therapeutics, we explore the latest in Cancer Immunology Research. The study highlights the promising preclinical data demonstrating the potential of TCR-T cell therapy in combination with lymph node-targeted Amphiphile-Immunotherapy to enhance anti-tumour function and eradicate solid tumours.

Can you elaborate on the key findings of the preclinical data published in Cancer Immunology Research? How significant are the results in terms of advancing TCR-T cell therapy and AMP immunotherapy for the treatment of solid tumours?
In this study, we demonstrated that AMP immunotherapy can boost TCR-T cell therapy by acting directly in the lymph nodes to promote the coordination of tumour-specific T cell responses by antigen presenting cells. This combination approach resulted in durable anti-tumour T cell responses and tumour eradication. Importantly, this was demonstrated in tumour models which were resistant to TCR-T cell monotherapy.
This combination approach resulted in durable anti-tumour T cell responses and tumour eradication.
We showed that AMP immunotherapy can synergise with tumour antigen-specific T cells to promote TCR-T cell expansion, persistence, and functional quality – and doing this directly in lymph nodes with AMP appears to be essential for driving that synergy, stimulating broad immune activation to invigorate both adoptive and endogenous anti-tumour T cell immunity.
We have already applied this approach to several important tumour targets where enhancements in TCR-T therapy may lead to important gains in clinical benefit, including mutated KRAS, NY-ESO, and HPV E7 antigens. Together, this suggests that AMP immunotherapy can be a very flexible strategy for promoting the efficacy of TCR-T cell therapy as a powerful new combination approach for many previously intractable solid tumours.
The study mentions the association of long-term protection against tumour recurrence in AMP-treated mice with antigen spreading to additional tumour-associated antigens. How does this mechanism contribute to the efficacy of the combination AMP immunotherapy strategy, especially in tumours refractory to TCR-T cell monotherapy?
AMP immunotherapy enhanced the infiltration and function of TCR-T cells in the tumour microenvironment and led to epitope spreading against diverse tumour targets. Long-term protection against tumour recurrence in AMP-treated mice was associated with antigen spreading to additional tumour-associated antigens not targeted by the treatment.
This antigen-spreading is critical to long-term protection because tumour often develop with inconsistent antigen expression – equipping the immune response to target many diverse tumour antigens may allow for more complete anti-tumour activity. It may also overcome resistance mechanisms developed by tumours to evade the immune response over time. If this AMP and TCR-T combination treatment provides an immune response against additional antigens, patients will potentially have deeper and more durable long-term responses to treatment, ultimately leading to better clinical outcomes.
The AMP platform is highlighted as delivering investigational immunotherapeutic directly to the lymph nodes. How does this site-specific delivery impact the immune response, and what advantages does it offer over immunotherapies that do not engage the lymph nodes?
The lymph nodes are critical for orchestrating the mechanisms which define tumour-directed immune responses. Here, we wanted to ask whether delivery to lymph nodes was important for allowing vaccines to (1) coordinate the expansion and functional qualities of tumour-specific TCR-T cells and (2) more broadly promote immune activation which could contribute to improved anti-tumour effect.
Many traditional vaccines and immunotherapies don’t target the lymph node – because they are often small in size, they are distributed from the injection site through the bloodstream, circulating to irrelevant sites or even tolerance-promoting organs (such as the liver) which can lead to toxicity or counterproductive immune suppression.
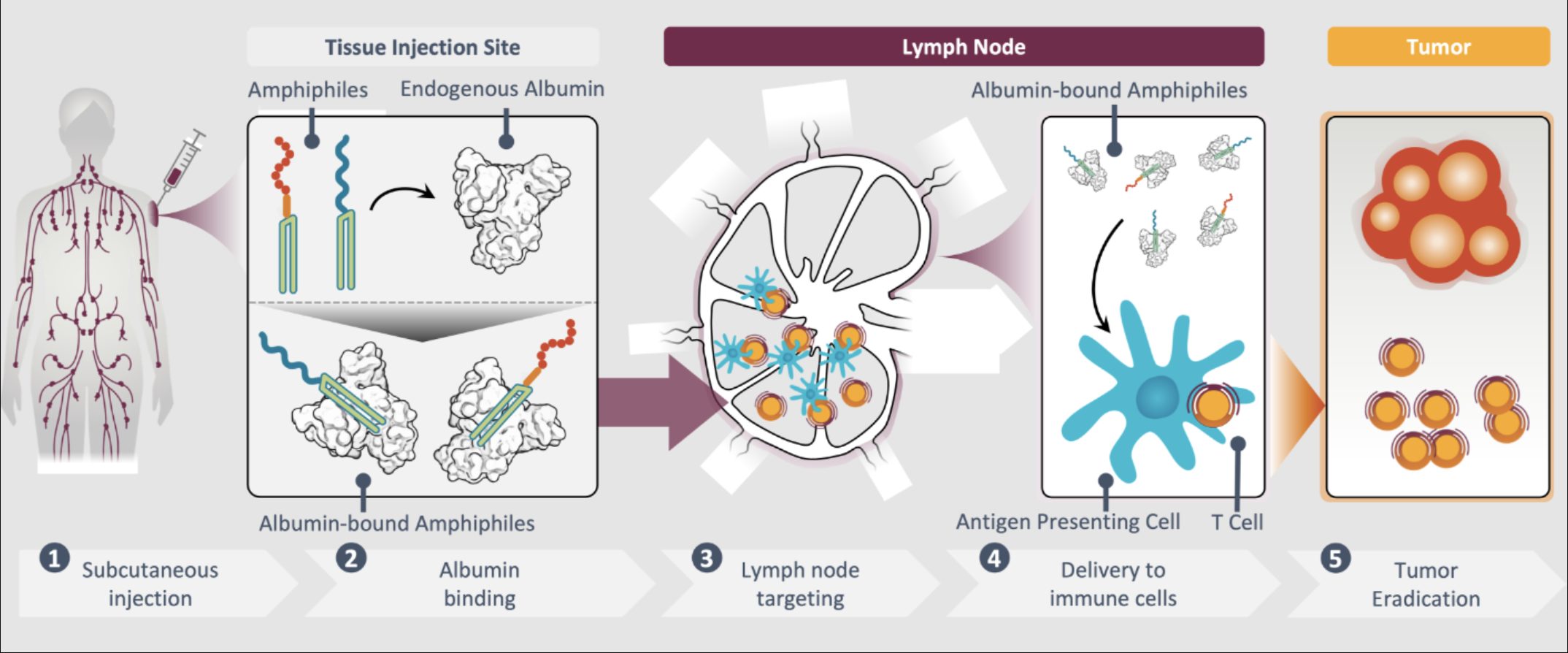

There, AMP-immunotherapies ‘educate’ T cells to recognise specific tumour antigens and eliminate those cancer cells.
We have conducted side-by-side studies comparing traditional soluble vaccines, which are known to have poor lymph node delivery, to our AMP vaccines to assess the importance of the lymph nodes for driving improvements in overall tumour immunity, and anti-tumour efficacy. Consistently, across our studies, the soluble vaccine comparators provided no benefit, while the analogous AMP therapies were able to invigorate the TCR-T cells, and enhance other mechanisms of host immunity, leading to improved anti-tumour function in mouse and human models of disease.
There is mention of the AMP platform’s potential application to clinical and developmental TCR-T cell programs. What considerations are important when exploring such combinations, and how does the AMP platform’s versatility make it a promising candidate for a wide range of cancer targets, including mKRAS, HPV E7, and NY-ESO-1?
Our AMP platform is built on simple chemistry designed to deliver a variety of therapeutic payloads to the lymph nodes in a way that is flexible and versatile.
To demonstrate this, we’ve applied this chemistry to the known peptide antigens targeted by TCR-T therapies in development for several well-known tumours antigens: NY-ESO-1, HPV E7, and mKRAS. TCR-T therapies specific to many other promising candidate antigens are in various stages of clinical testing or development, and complimentary AMP-peptides can be designed and manufactured quickly based only on the sequence of the peptide antigen targeted by the TCR. These could then be administered in combination with the TCR-T therapy to promote their expansion, anti-tumours activity, and persistence. Existing TCR-T therapies have shown great promise, but challenges like limited persistence of the TCR-Ts and poor trafficking into the solid tumours remain – this strategy has the potential to overcome many of these challenges where TCR-T therapies have not achieved the consistent or durable activity needed to provide adequate clinical benefit to patients.
About the author


Executive Vice President, Head of Research and Development, and Chief Medical Officer of Elicio
Dr Haqq is an oncologist with more than two decades of experience in drug development working across cell therapy, small molecule and biologics in large and small biotech settings. Dr Haqq holds multiple patents and publications. He has served as the medical monitor for numerous oncology clinical trials and has worked closely with the US Food and Drug Administration and other global regulatory agencies to advance therapies through the clinic to marketing approval.
Related topics
Drug Delivery, Drug Development, Immuno-oncology, Immuno-oncology therapeutics, Immunology, Immunotherapy, Targets
Related conditions
Cancer, Cancer Research
Related organisations
Elicio Therapeutics
Related people
Dr Christopher Haqq (Elicio)








