Beyond templates: advancing protein–protein interaction structure prediction with AI
Posted: 3 October 2025 | Dr Alan Nafiiev (CEO and Founder of Receptor.AI) | No comments yet
Dr Alan Nafiiev evaluates template-based, docking and template-free approaches to PPI prediction, highlighting how AI can enhance structural accuracy.

Introduction
Protein–protein interactions (PPIs) are the specific contacts formed when two proteins bind. Understanding their three-dimensional structures is essential for designing compounds that can modulate PPIs, which is the most advantageous strategy for treating many diseases.1 However, experimentally determining PPI structures is slow, costly and often technically challenging.
Currently, the primary computational approach for PPI structure prediction has been protein–protein docking, where known protein structures are positioned to identify a plausible interface. This method remains fundamentally limited because it treats proteins as rigid bodies and fails to account for solvent effects, side-chain rearrangements, backbone flexibility and other biophysical factors.2
Now, artificial intelligence (AI) is poised to break through these limits.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
Template-based PPI structure prediction
Template-based PPI prediction methods assemble complexes by taking two target sequences, finding a homologous complex in a structural database, and ‘grafting’ the known backbone and interface onto the new pair. When a close template exists, this can yield remarkably accurate interfaces in minutes. Recent generative AI models, such as AlphaFold-Multimer or RoseTTAFold, also draw on these same resolved complexes as part of their training data, but go further by integrating deep multiple‐sequence alignments and co-evolutionary signals.3
Yet the template library itself remains woefully sparse. BioGRID curates evidence for over 1.4 million human PPIs,4 yet only a tiny fraction, just 4,594 complexes, have high-resolution structures in PDBbind-plus – a database designed to offer a comprehensive collection of experimental binding affinity data for biomolecular complexes.5 This means templates cover under 1 percent of the estimated human interactome, even adding unique structures from other sources. Moreover, the complexes we do know are heavily biased towards stable, soluble, globular assemblies, while most cellular interactions are transient, involve intrinsically disordered regions, or occur at membrane surfaces. Because template-based methods depend directly on the available structural data, their accuracy collapses outside this narrow subset.
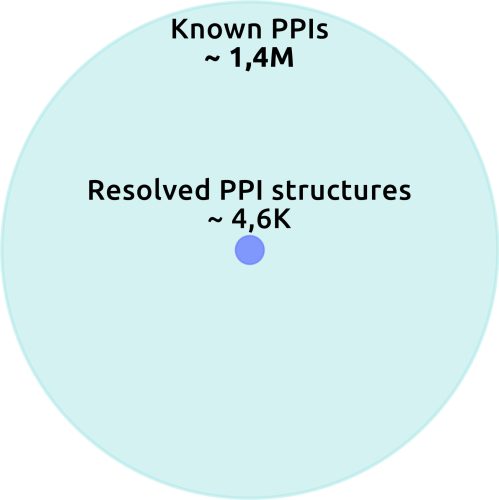

Figure 1. The proportion of resolved PPI complexes among known PPIs. Credit: Receptor.AI
Template-free PPI structure prediction
With an example of Receptor.AI’s technology DeepTemplateAGnostic (DeepTAG), an alternative approach can be described. Template‐free PPI prediction takes a fundamentally different tack: instead of searching for a matching scaffold, it first scans each protein surface to locate ‘hot-spots’ – clusters of residues whose side-chain properties (size, hydrophobicity, charge potential and solvent exposure) favour binding.6
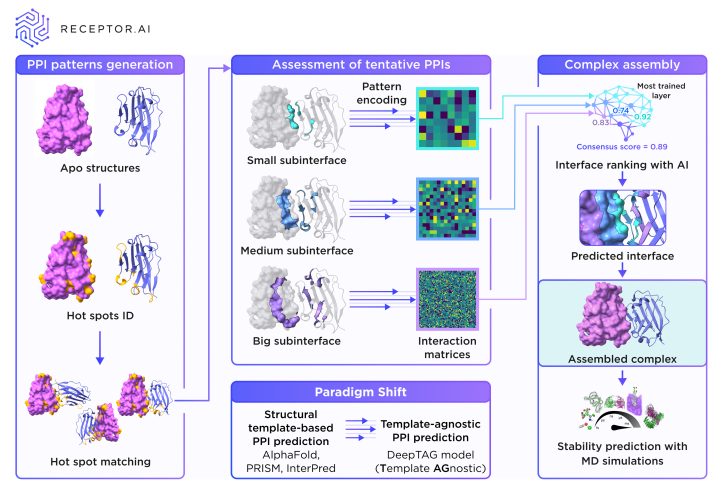

Figure 2. DeepTAG workflow. Candidate interfaces are split onto three sub-interfaces and scored by different AI layers with different training data. Consensus score is then determined. Credit: Receptor.AI
Once hot-spots on each partner are identified, hot-spot matching is performed to define a limited set of candidate interfaces. For each candidate, contact matrices are constructed that describe which residues on protein A lie within binding distance of residues on protein B.
Here, machine learning excels by using models trained on residue-to-residue contacts within folded domains to score each inter-protein interaction matrix for its predicted binding energy. Because monomeric structures are more highly researched than protein complex structures, this method also works for complexes far from similar to known.
With the best-scored interface defined, the rest of the complex is built around it and the full assembly is tested for stability using molecular dynamics simulations. As the PPI interface itself is the primary target for drug design, ensuring its accuracy, even if other regions are modelled with less precision, is sufficient for downstream drug discovery efforts.
Benchmark results
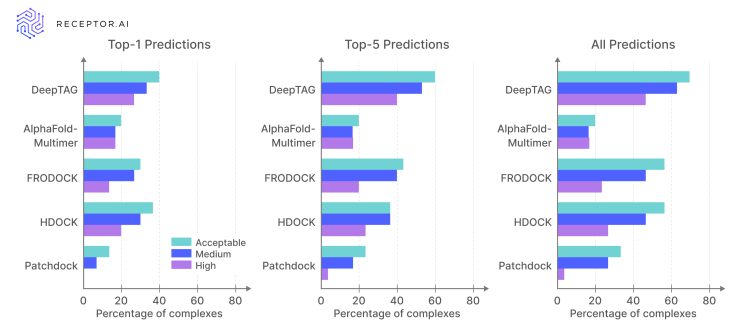

Figure 3. Comparison of docking and AI methods on the PINDER-AF2 benchmark (30 unbound pairs). Bars show the percentage of complexes achieving Acceptable, Medium, or High DockQ accuracy for each method’s Top-1, Top-5 and All predictions. Credit: Receptor.AI
To objectively compare template-based, docking and template-free workflows, we tested them in a standardised benchmark of challenging targets – PINDER-AF2. Its dataset comprises 30 protein–protein complexes provided only as unbound monomer structures, mirroring real-world scenarios where no prior complex is available. Each method was tasked with generating complexes that were then evaluated by the CAPRI DockQ metric, which scores structural similarity to the native complex on a scale where 0.23–0.49 is Acceptable, 0.49–0.80 is Medium, and above 0.80 is High.7 Results are reported for the Top-1 prediction, the best among the Top-5, and across all predictions.
Template-based prediction, exemplified by AlphaFold-Multimer, performs worse than classic rigid-body docking with HDOCK. AlphaFold-Multimer’s metrics barely change when you expand from Top-1 to All predictions, meaning the model simply fails to predict enough high-quality interfaces.
In contrast, template-free prediction already outperforms protein–protein docking even in the Top-1 results. Moreover, the plots show that DeepTAG generates an even larger share of high-quality complexes (nearly half of all candidates reach ‘High’ accuracy), yet not all of them were ranked higher by the model. With ongoing work on improving scoring, the model’s performance in real drug discovery will be truly unmatched.
Conclusion
In conclusion, template-free PPI prediction sidesteps the limits of template scarcity by focusing on protein surface hot-spots. DeepTAG already outperforms protein–protein docking in accuracy and, with ongoing scoring improvements, promises truly unmatched support for PPI targeting in drug discovery. AI is a powerful tool but not a one-size-fits-all solution – it must be applied thoughtfully and in the right context.
Meet the author


References
- Singh A, Nguyen B, Ng HL. Editorial: Prediction of protein-protein interactions (PPIs): the next frontier. Frontiers in Molecular Biosciences. 2024;11. doi: 10.3389/fmolb.2024.1479705
- Desta IT, Porter KA, Xia B, et al. Performance and its limits in rigid body protein-protein docking. Structure. 2020;28(9):1071-1081.e3. doi: 10.1016/j.str.2020.06.006
- Evans R, O’Neill M, Pritzel A, et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv. 2021. doi: 10.1101/2021.10.04.463034
- BioGRID. Statistics. The Biological General Repository for Interaction Datasets. Accessed August 15, 2025. https://wiki.thebiogrid.org/doku.php/statistics
- PDBbind-Plus database. Accessed August 15, 2025. https://www.pdbbind-plus.org.cn/
- Chen YC, Sargsyan K, Wright JD, et al. PPI-hotspotID for detecting protein–protein interaction hot spots from the free protein structure. eLife. 2024;13:RP96643. doi: 10.7554/eLife.96643.3
- Basu S, Wallner B. Finding correct protein–protein docking models using ProQDock. Bioinformatics. 2016;32(12):i262-i270. doi: 10.1093/bioinformatics/btw257
Related topics
Analysis, Artificial Intelligence, Bioinformatics, Computational techniques, Drug Discovery, Drug Discovery Processes, Molecular Modelling, Protein, Structural Biology, Translational Science
Related organisations
Receptor.AI
Related people
Dr Alan Nafiiev (CEO and Founder of Receptor.AI)