The other end of the antibody: Fc biology and immunotherapy
Posted: 14 June 2021 | Dr Björn Frendeus (BioInvent) | 1 comment
Dr Björn Frendeus outlines how the growing biology surrounding the inhibitory Fc receptor FcγRIIb defines a target for improving existing and future antibody treatments.
Over 50 antibody drugs have now been approved for the treatment of various types of cancer.1 They have made major contributions to increased survival rates seen in haematological and solid cancers. Many antibodies used in oncology have become highly commercially successful despite the incomplete understanding of molecular and cellular control mechanisms that could contribute to an effective and long-lasting anticancer response. However, only in the last 10 years or so have antibody drugs individually engineered for improved fragment crystabllisable (Fc) function reached the market. Their clinical success may herald the emergence of approaches designed to enhance the performance of antibody drugs as a class. This article highlights the potential role of the sole human inhibitory Fcγ receptor, FcγRIIb, in boosting the activities of antibody drugs.
In evolutionary terms, interactions between antigen-bound antibodies and Fc receptors link adaptive immunity to the pattern-recognising innate immune system. While the antigen-binding fragment variable (Fv) region determines the target specificity of the antibody, Fc regions and the Fc receptors on the surfaces of myeloid immune cells and macrophages play a major role in determining the consequences of antigen-antibody binding. In effect, antibodies have two targets: the antigen at one end and the Fc receptor at the other. While therapeutic antibodies are usually selected to bind a single antigen with high affinity, the binding between the Fc and its receptor is a low affinity, high avidity interaction.
Since all antibodies approved as cancer therapeutics are Immunoglobulin G (IgG) molecules, it is Fcγ receptors (FcγR) that are of particular interest in oncology. Humans have several activating FcγRs (FcγRI, FcγRIIa, FcγRIIc and FcγRIIIa) but only one inhibitory receptor, FcγRIIb. Mice have a similar arrangement of multiple activating, but a single inhibitory, receptor, perhaps reflecting a biological requirement to be able to raise and direct an immune response in a nuanced, multidimensional manner while retaining a means of shutting it down via a single braking mechanism.
Fcγ receptors are important potential targets in autoimmunity (some destruction and inflammation wrought by autoantibodies is thought to occur in a FcγR-dependent manner). A recent Nature paper2 has suggested that IgG subclass selectivity may influence the severity of COVID-19 infections and that antibodies that block FcγRIIb might help address this. However, this article will focus on the importance of Fcγ receptors as targets in modulating the actions of antibody drugs as cancer treatments.
When the earliest antibodies were approved for use in cancer immunotherapy, understanding of the importance of Fcγ receptors was, at best, incomplete. Rituxan (rituximab), a CD20-binding antibody, was approved in 1997, followed shortly by the Her2-binder Herceptin (trastuzumab) in 1998 and a few years later in 2004 by Erbitux (cetuximab) for EGFR-expressing cancers.
 By the beginning of the 2000s, however, rituximab had been around long enough as a lymphoma treatment for physicians to become aware that not all patients responded in the same way to the drug. Non-Hodgkin’s lymphoma (NHL) patients who are homozygous for a high-affinity variant of the activating FcγRIIIa gene, for instance, did much better on rituximab than those who carried a gene variant for a low-affinity variant: their times-to-disease-progression were significantly longer.3 Enhanced survival similarly correlated with high affinity variants of FcγRIIa, another activating receptor.4 The physicians noted that it might be useful to collect information on genotypic variation in future rituximab trials, but beyond that, there was not much to be done for patients with a less responsive genotype.
By the beginning of the 2000s, however, rituximab had been around long enough as a lymphoma treatment for physicians to become aware that not all patients responded in the same way to the drug. Non-Hodgkin’s lymphoma (NHL) patients who are homozygous for a high-affinity variant of the activating FcγRIIIa gene, for instance, did much better on rituximab than those who carried a gene variant for a low-affinity variant: their times-to-disease-progression were significantly longer.3 Enhanced survival similarly correlated with high affinity variants of FcγRIIa, another activating receptor.4 The physicians noted that it might be useful to collect information on genotypic variation in future rituximab trials, but beyond that, there was not much to be done for patients with a less responsive genotype.
At that time, at least three potential mechanisms were widely discussed: the engagement of FcγRs by the Fc regions of antigen-bound antibodies was one of them, but the activation of the complement pathway or antibody-triggered tumour cell apoptosis were each highly plausible based on the in vitro and in vivo studies that had been performed at that point.
Differential activation of the complement pathways was largely discounted after several studies5,6 indicated that mice deficient in complement pathways could nevertheless mount potent anticancer responses.
While many research groups were occupied either in a search for new molecular targets in oncology against which to direct antibodies, or striving to find novel antibody modalities that could help circumvent intellectual property on known oncology targets, Jeffrey Ravetch and colleagues at the Laboratory of Molecular Genetics and Immunology at Rockefeller University adopted a systematic approach for dissecting the interactions between antibody Fc regions and their Fcγ receptors.
Anticancer antibodies are no longer late-line exotic additions to cancer therapy”
The Ravetch group showed that while that the cross-linking of activating FcγRs expressed on myeloid cells results in sustained cellular responses, co-ligation of the inhibitory receptor, FcγRIIb, reduces that cellular response.7 They also noted that that the binding affinities of different FcγRs for IgG subclasses varied markedly8 and used this observation to calculate an activating-to-inhibitory (A/I) ratio for each IgG subclass. Then in a seminal paper9 in Science in 2005, Ravetch and Falk Nimmerjahn demonstrated the consequences of the A/I ratio. Firstly, they ‘grafted’ identical antigen-binding regions onto IgG-subclass Fc regions and showed that the binding affinities for FcγRs and their expected biological activities remained associated with the antibody subclass. Using knock-out mice, they showed clear enhancement of anticancer activity in mice deficient in the inhibitory FcγRIIb.
The conclusion from this work9 was that the therapeutic effects of antibodies are modulated by effector cells (myeloid cells, macrophages) and that efficacy can be optimised by fine-tuning antibody interactions with activating and inhibitory Fcγ receptors. While efficacy of most clinically approved anticancer antibodies (eg, anti-CD20, anti-Her2, anti-EGFR and anti-CTLA-4) are enhanced by FcγR-dependent mechanisms, there are important exceptions depending on target biology and expression profile. Complementary approaches of fine-tuning FcγR-interactions will therefore be needed to improve efficacy of therapeutic antibodies with different mechanisms of action. For therapeutic antibodies whose activity is enhanced by engaging in phagocytosis or other tumour cells deleting mechanisms, they must have an appropriate A/I ratio – sufficiently high numbers of activating Fc receptors and relatively low numbers of inhibitory receptors.
With an understanding of the biology of Fc-FcγR interactions, two main avenues for drug development open up: either optimise binding to activating FcγRs or reduce binding to the lone inhibitory FcγR, FcγRIIb. Both strategies have been adopted by a number of biotechnology and pharmaceutical firms.
Enhancing activating FcγR
 There are a number of routes to enhancing activating FcγR interactions and many involve changing the molecular composition of the antibody molecule. While this has commercial benefits in creating new intellectual property, it also invokes a need for extensive pre-clinical and clinical testing. Several molecules have emerged from that process.
There are a number of routes to enhancing activating FcγR interactions and many involve changing the molecular composition of the antibody molecule. While this has commercial benefits in creating new intellectual property, it also invokes a need for extensive pre-clinical and clinical testing. Several molecules have emerged from that process.
For IgG1 antibodies, one conceptually simple ‘trick’ that sprang from ETH Zurich10 was the development of an engineered Chinese hamster ovary (CHO) cell line that produced antibodies without fucose in the oligosaccharides in the Fc region. Afucosylation maximises the binding affinity of IgG1 for FcγRIIIa on natural killer (NK) cells. Hoffmann-La Roche bought the ETH spin-out GlycArt in 2005 in order to acquire the afucosylating technology, launching Gazyva (obinutuzumab), the first product from the acquisition in 2013. Kyowa Hakko Kirin subsequently developed a similar CHO cell process11 that led to the development of Poteligeo (mogamulizumab), launched in Japan in 2012.
The potency of anticancer antibodies can also be improved by changing the amino acid composition of the Fc region. US biotech Xencor changed two amino acid residues in the Fc region of an anti-CD19 antibody nearly doubling the level of activation of NK cells and greatly increased NK cell-mediated antibody-directed cellular cytotoxicity in vitro.12 That antibody, Morphosys’ Monjuvi (tafasitamab-cxix), received marketing approval from the US Food and Drug Administration (FDA) in July 2020.
Another Fc-engineered monoclonal antibody (mAb) was approved in December 2020: Margenza (margetuximab-cmkb) is targeted at Her2. To create margetuximab, researchers at Macrogenics used an Fc-engineering approach to alter five amino in a wild-type anti-Her2 antibody. They both reduced the Fc affinity for the inhibitory FcγRIIb and increased affinity for the activating FcγRIIIa. In a head-to-head clinical study, margetuximab outperformed Herceptin (trastuzumab) in the treatment of advanced breast cancer, reducing the risk of disease progression and increasing the time for overall survival.13 The relative contributions to clinical performance of increased FcγRIIIa activation and reduced FcγRIIb inhibition were not disclosed.
Reducing inhibitory FcγR
Many antibodies used in oncology have also become highly commercially successful”
Rather than increasing activation of activating FcγRs, another strategy is to reduce the inhibitory effects of the inhibitory FcγR, FcγRIIb. In principal, this represents a broader opportunity since, unlike the activating receptors, polymorphic variation in FcγRIIb is very limited. Furthermore, while the expression of the various activating Fcγ receptors on effector cells can differ from person to person and in different tissues, the counterbalance to this variegated pattern of activating FcgRs is always FcγRIIb, the single inhibitory receptor that is upregulated in intratumoural compartments compared with blood.
The proof-of-principle for this approach was established by the Ravetch group.14 They showed that deletion of the inhibitory FcγR in mice boosts the anticancer activity of antibodies to several clinically validated targets such as anti-CD20 and anti-Her2.
While genetic deletion of FcγRIIb is not an option in humans, the alternative – to block the inhibitory FcγRIIb with an antibody – requires a nuanced technical approach. The first challenge is that the antibody-accessible domain of FcγRIIb is around 93 percent homologous with the activating receptor, FcγRIIa. A second challenge is the need simply to block FcγRIIb rather than trigger FcγRIIb-mediated inhibitory signalling.
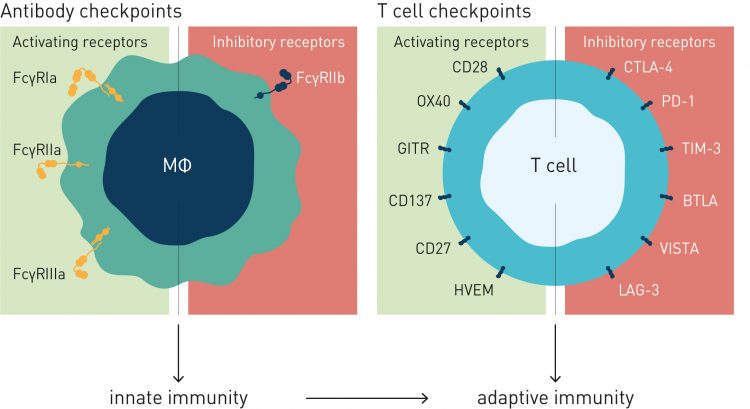
BioInvent screened a large and diverse recombinant antibody library and found most FcγRIIb-specific antibodies also activated FcγRIIb phosphorylation, which excluded them from consideration. It was possible, however, to isolate two antibodies that blocked FcγRIIb inhibitory signalling without activating FcγRIIb phosphorylation. One of these is now in Phase I development for the treatment of NHL and solid tumors, while the other is under pre-clinical investigation.15
There is increasing evidence that similar mechanisms could be invoked for the enhancement of T-cell checkpoint inhibitor antibodies targeted at CTLA-4 (eg, ipilimumab) or IL-2R, where the target cells can be considered to include intratumoral regulatory T cells.16
Blocking FcγRIIb is of specific importance in lymphoma where the inhibitory receptor is expressed both on the effector B cells that help eliminate tumour cells and on the tumour cells themselves. The presence of FcγRIIb on B cells or lymphoma cells can trigger the internalisation of therapeutic antibodies such as rituximab,17 in effect ‘mopping up’ the targeting antibody. A series of FcγRIIb-blocking antibodies reduced rituximab internalisation to an extent that directly correlated with the affinity of the antibodies for the inhibitory receptor.15
Anticancer antibodies are no longer late-line exotic additions to cancer therapy but a core part of mainstream oncology treatment. Novel antigens will continue to emerge and treatment protocols will be increasingly tailored to personal cancer profiles. In parallel, an increased focus on the ‘other end’ of the antibody could bring an element of systems engineering into immunobiology and allow oncologists to use both new and existing products more effectively.
About the author
Dr Björn Frendéus, is the Chief Scientific Officer of BioInvent, a Swedish biotech developing antibody-based treatments for cancer immunotherapy. Björn is a Doctor of Immunology, and a visiting professor at the Cancer Sciences Division in Southampton, UK. Björn chairs the Swedish Foundation for Strategic Research’s expert review committee on Infection Biology.
References
- Kaplon H, Reichert JM. Antibodies to watch in 2021. MAbs. 2021 Jan-Dec;13(1):1860476. doi: 10.1080/19420862.2020.1860476.
- Combes, A.J., Courau, T., Kuhn, N.F. et al. Global absence and targeting of protective immune states in severe COVID-19. Nature 591, 124–130 (2021). https://doi.org/10.1038/s41586-021-03234-7
- Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. (2002) 99:754–8. doi: 10.1182/blood.V99.3.754
- Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003 21(21):3940-7. doi: 10.1200/JCO.2003.05.013.
- Sylvestre D, Clynes R, Ma M, et al. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J Exp Med. 1996; Dec 1;184(6):2385-92. doi: 10.1084/jem.184.6.2385.
- Clynes R, Ravetch JV. Cytotoxic antibodies trigger inflammation through Fc receptors. Immunity. 1995 Jul;3(1):21-6. doi: 10.1016/1074-7613(95)90155-8.
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275-90. doi: 10.1146/annurev.immunol.19.1.275.
- Nimmerjahn F, Bruhns P, Horiuchi K, and Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005 23(1):41-51. doi: 10.1016/j.immuni.2005.05.010.
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005 310 (5753):1510-2. doi: 10.1126/science.1118948.
- Umaña P, Jean-Mairet J, Moudry R, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999 Feb;17(2):176-80. doi: 10.1038/6179.
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004 87(5):614-22. doi: 10.1002/bit.20151.
- Kellner C, Zhukovsky EA, Pötzke A, et al. The Fc-engineered CD19 antibody MOR208 (XmAb5574) induces natural killer cell-mediated lysis of acute lymphoblastic leukemia cells from pediatric and adult patients. Leukemia. 2013 27(7):1595-8. doi: 10.1038/leu.2012.373.
- Rugo HS, Im SA, Cardoso F, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021 Jan 22:e207932. doi: 10.1001/jamaoncol.2020.7932.
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000 6(4):443-6. doi: 10.1038/74704.
- Roghanian A, Teige I, Mårtensson L, et al. Antagonistic human FcγRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell. 2015 Apr 13;27(4):473-88. doi: 10.1016/j.ccell.2015.03.005.
- Arce Vargas F, Furness AJS, Litchfield K, et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell. 2018 Apr 9;33(4):649-663.e4. doi: 10.1016/j.ccell.2018.02.010.
- Lim SH, Vaughan AT, Ashton-Key M, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011 Sep 1;118(9):2530-40. doi: 10.1182/blood-2011-01-330357.
Related topics
Antibodies, Immuno-oncology, Immunotherapy, Oncology, Protein, Proteomics
Related conditions
Cancer, lymphoma, Non-Hodgkin’s lymphoma (NHL)
Related organisations
BioInvent, ETH Zurich, Rockefeller University, US Food and Drug Administration (FDA), Xencor
Related people
Falk Nimmerjahn, Hoffmann-La Roche, Jeffrey Ravetch, Kyowa Hakko Kirin







The article gives a very clear overview of the Fc biology especially emphasizing on the role of inhibitory Fc receptor FcγRIIb.