Using brain models of patient-specific Alzheimer’s insights
Posted: 23 September 2021 | Victoria Rees (Drug Target Review) | No comments yet
In a new study, researchers at Brigham and Women’s Hospital, US, successfully developed stem cell-derived neuronal profiles from individual patients. Here, Drug Target Review’s Victoria Rees explores the findings and how these new models can help to advance precision and personalised medicine.

Alzheimer’s disease is the most common form of dementia and causes memory loss and reduced cognitive skills.1 The build-up of certain proteins can trigger the onset of the disease and result in Alzheimer’s pathogenesis. However, with a higher risk of development in older patients, it can sometimes be difficult to identify the condition in its early stages.
According to official records, over 120,000 people died as a result of Alzheimer’s disease in the US in 2019. This number makes Alzheimer’s the sixth‑leading cause of death and the fifth‑leading cause of death among those aged 65 and older in the US.2
To reduce the number of Alzheimer’s deaths per year, novel treatment pathways and strategies must be identified. New research led by scientists at Brigham and Women’s Hospital has aimed to solve this issue on an individualised level.
In a paper published in Neuron,3 the team from the study explain how they have established a resource for exploring and understanding Alzheimer’s disease. The researchers generated induced pluripotent stem cell (iPSC) lines from over 50 different individuals for whom longitudinal clinical data, quantitative neuropathology data and rich genetic and molecular profiling of brain tissue was also available. By turning these iPSCs into brain cells, the researchers were then able to analyse the molecular pathways involved in the disease and reveal which classes of proteins were associated with cognitive decline.
Predicting outcomes of Alzheimer’s
According to the researchers, the variation of genetic backgrounds of humans generates unique profiles of the amyloid beta-protein (Aβ) and tau protein, meaning their presence is different in each person.
While forms of these proteins have long been associated with cognitive decline and Alzheimer’s disease, the signalling pathways that influence the production of these toxic species have not been well defined, especially between different individuals. The team thus developed their stem cell-derived neuronal profiles to be predictive of individual clinical outcomes of Alzheimer’s disease.
“This large set of human cell lines from Alzheimer’s disease and cognitively normal people will provide the scientific community with a powerful experimental system for untangling why some people develop Alzheimer’s disease and others do not,” said Dr Tracy Young-Pearse of the Brigham’s Division of Neurology and one of the study’s authors.
Developing the neural cells
The study leveraged a large cross-institutional collaborative effort that follows a cohort of people from the Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP) at Rush University, US. All individuals entered the ROS and MAP studies with no history of Alzheimer’s disease or any other neurological diagnosis, but over one third of individuals developed Alzheimer’s disease over time. Stem cell lines were generated from blood samples of over 50 of these individuals, who lived to an average of 90 years of age. While the subjects were alive, detailed clinical records and full genome sequencing were collected for each person. After death, the brains of the deceased also were studied and compared to their cultured brain cells in a dish.
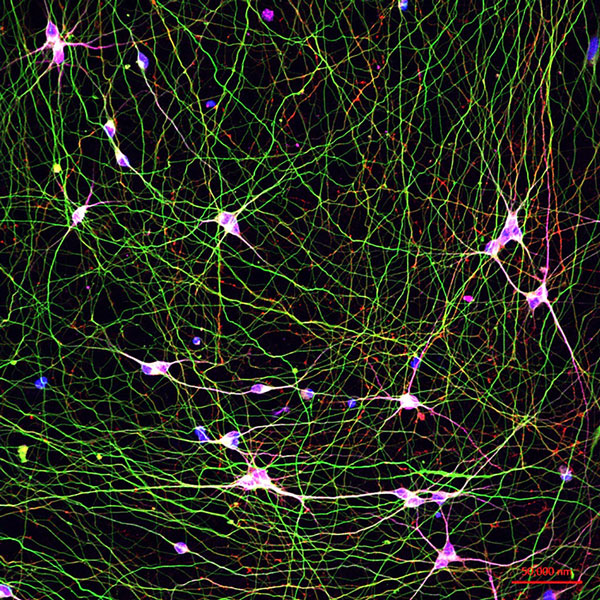

Human stem cell-derived neurons in a dish [credit: Young-Pearce lab].
The team from the study turned the human stem cells into brain cells and then analysed the molecular pathways within the living neurons in vitro to identify which ones were active. RNA and proteomic profiling of both iPSC-derived neurons and brain tissue of the same individuals was conducted. Further data were collected via genome sequencing, longitudinal cognitive scores and quantitative neuropathology.
They measured Aβ and tau generated by the patient stem cell-derived neurons and found that specific species of these proteins were associated with Alzheimer’s levels of plaque and tangle deposition in the brain and the trajectory of cognitive decline.
“If you look across cell culture samples from 50 people, you can predict from the Aβ and tau profiles some features of the cognitive status of that person, their rate of cognitive decline and whether they developed Alzheimer’s disease, which is absolutely remarkable,” said Young-Pearse.
Next steps for the Alzheimer’s study
The authors emphasise that, since participants of the ROS and MAP cohorts were primarily Caucasians of European origin, their observations may not be applicable to more diverse populations. The research team has already undertaken the development of 50 new iPSC lines which will be derived from non-Caucasian groups.
“This is a resource for the whole community, for scientists around the world to use,” said Young‑Pearse. “We already have around 50 labs, all around the world, starting to use these cells in their studies.”
The research team is also exploring if the stem cell-derived neurons can be used to predict whether drugs like aducanumab, a new drug recently approved by the US Food and Drug Administration (FDA) to treat Alzheimer’s disease, will be more effective in specific groups of patients with Alzheimer’s.
“The new Alzheimer’s drug is an antibody that recognises Aβ and clears it from the brain. Our work shows that different genetic backgrounds of human neurons produce different profiles of Aβ,” said Young-Pearse. “What our system provides is a platform to test who might be responsive to different Alzheimer’s disease therapeutics, for example, anti-Aβ and anti-tau immunotherapeutics. This will be important because neurons from different individuals produce different profiles of Aβ and tau and different antibody cocktails may be more effective against one profile over another.”
The underlying causes of Alzheimer’s
The research team further conclude that Alzheimer’s disease is not caused by a single genetic mutation but instead can have different sets of genes contributing to risk across different people. This means that the primary causes of Alzheimer’s disease can be varied and therefore treatments may also need to be tailored to the underlying causes of disease.
“There is a perception that this disease is one uniform disease that shows up and progresses the same way for everyone,” Young-Pearse said. “It is important to understand that Alzheimer’s is actually quite heterogeneous in its underlying causes, ages of onset and disease course. This is the first time we have a system in place to study living human brain cells from many people to understand better why some develop Alzheimer’s disease in a very specific way and others are resistant to the disease.”
Conclusion
In summary, the iPSC-derived neuron models developed by the researchers could help to progress precision and personalised medicine by evaluating the best treatment course for patients. With accurate predictions of disease outcomes, healthcare professionals and patients could be advised of the most effective therapeutic regimes. Likewise, this new development could also help researchers to further investigate the underlying causes of Alzheimer’s disease, in the hope that a broad acting treatment could one day be approved and in use.
About the author
Victoria Rees is the Deputy Editor of Drug Target Review.
References
- What is Alzheimer’s disease? | Alzheimer’s Research UK [Internet]. Alzheimer’s Research UK. 2021 [cited 9 September 2021]. Available from: https://www.alzheimersresearchuk.org/dementia-information/types-of-dementia/alzheimers-disease/
- 2021 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2021;17(3):327-406.
- Lagomarsino V, Pearse R, Liu L, Hsieh Y, et al. Stem cell-derived neurons reflect features of protein networks, neuropathology and cognitive outcome of their aged human donors. Neuron. 2021.
Related topics
Analysis, Disease Research, Genetic Analysis, Induced Pluripotent Stem Cells (iPSCs), Neurosciences, Personalised Medicine, Precision Medicine, Stem Cells
Related conditions
Alzheimer's disease (AD)
Related organisations
Brigham and Women’s Hospital at Harvard Medical School, Religious Orders Study (ROS), Rush Memory and Aging Project (MAP), Rush University Medical Center
Related people
Dr Tracy Young-Pearse








