New insight into the key properties of an antibody needed to fight cancer
Posted: 11 July 2022 | Ria Kakkad (Drug Target Review) | No comments yet
The findings have enabled researchers to design antibodies to activate important receptors on immune cells and deliver more powerful anti-cancer effects.
Scientists at the University of Southampton, UK, have gained insight into the key properties of an antibody to fight against cancer. The study, which was recently published in Science Immunology, revealed how changing the flexibility of the antibody could stimulate a stronger immune response.
The findings have enabled the team to design antibodies to activate important receptors on immune cells and deliver more powerful anti-cancer effects.
In the study, the team investigated antibody drugs targeting the receptor CD40 for cancer treatment. Previous research has shown that a specific type of antibody called IgG2 is uniquely suited as a template for pharmaceutical intervention, since it is more active than other antibody types. However, the reason why it is more active had not been determined.
What was known, however, is that the structure between the antibody arms, the so-called hinges, changes over time. This latest research harnesses this property of the hinge and explains how it works: the researchers call this process ‘disulfide-switching’.
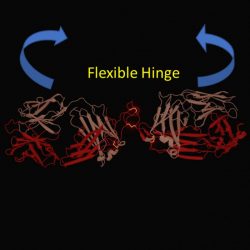
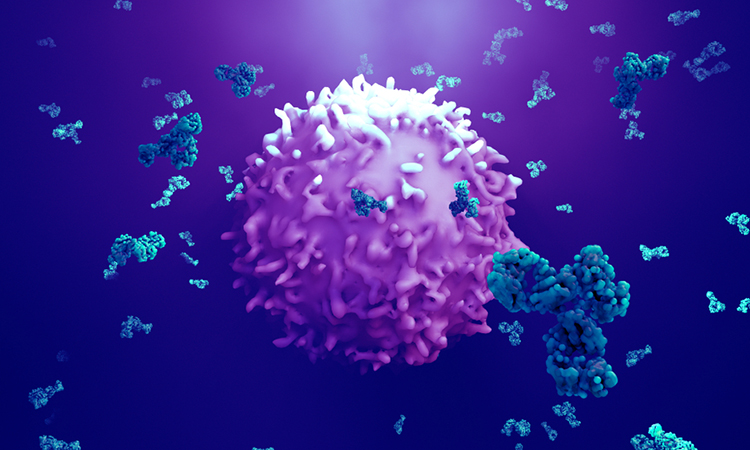
The flexible hinge allows the two antibody arms to move freely, leading to weak receptor activity
[Credit: University of Southampton].
In their study, the Southampton team analysed the effect of modifying the hinge and used a combination of biological activity assays, structural biology, and computational chemistry to study how disulfide switching alters antibody structure and activity.
“Our approach was to analyse the structure of the antibody in atomic detail, using the method of X-ray crystallography. While the resulting picture is very accurate, the information on how they move their ‘arms’ is missing, and we needed an image of the antibody in solution, for which we used an X-ray scattering approach called SAXS. We then used mathematical models and a chemical-computing approach to analyse the data, using the Southampton High Performance Computing cluster IRIDIS,” said Dr Ivo Tews.
Through this detailed study of the hinge the team revealed that more compact, rigid antibodies are more active than their flexible counterparts.
“This study has given us new information about how to engineer antibodies to deliver a better immune response. We propose that more rigid antibodies enable the receptors to be bound closer together on the cell surface, promoting receptor clustering and stronger signalling for activity. This means by modifying the hinge we can now generate more or less active antibodies in a more predictable way, said Professor Mark Cragg. “Excitingly, our findings could have wider implications as it may provide a highly controlled and tractable means of developing antibodies for clinical use in future immunostimulatory antibody drugs.”
Related topics
Antibodies, Antibody Discovery, Immunology
Related conditions
Cancer
Related organisations
Southampton University
Related people
Dr Ivo Tews, Professor Mark Cragg