Making lab-grown brain organoids ‘brainier’
Posted: 3 October 2022 | Ria Kakkad (Drug Target Review) | No comments yet
UCLA-led team discovers that using early-stage stem cells is a key to producing structures that are reliable models of disease.

Currently, organoids can vary from lab to lab — and even from batch to batch — as there is no common protocol for their production and a lack of quality-control guidelines, which means that a finding made in one organoid may not hold true in another. In their new study, published today in Stem Cell Reports, researchers from UCLA have proposed guidelines based on their research that can help scientists overcome two major obstacles standing in the way of organoids’ full potential: differences in uniformity and structure.
Having organoids that accurately and consistently recreate the structure and cellular makeup of specific sections of the brain is especially important for studying disorders like schizophrenia and autism spectrum disorder in which the brains of affected people often appear identical to neurotypical brains in structure yet exhibit marked differences in function.
To produce mini–brain organoids, which can range from 1 to 5 millimetres in diameter, scientists first take human skin or blood cells and reprogram them to become induced pluripotent stem (iPS) cells. They then direct these iPS cells to create neural stem cells, which can produce most cell types found in the brain. As the neural stem cells are forming, they can be coaxed to aggregate into three-dimensional organoids.
The researchers discovered that some organoids better resemble the human brain than others because the maturity of the stem cells from which an organoid is grown influences its quality.
“In human embryonic development, the nervous system is one of the first structures to form, so it makes sense that stem cells that are early in development are best at producing brain organoids,” said Assistant Professor Momoko Watanabe, the paper’s first author.
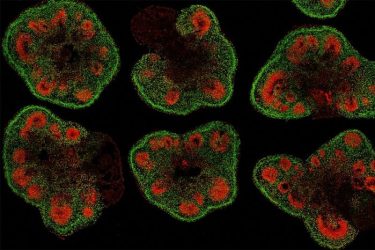

Slices of mini–brain organoids with neural stem cells (red) and cortical neurons (green)
[Credit: Hajime Ozaki, Watanabe lab/UCI].
The researchers then found that the best way to keep human stem cells in an early developmental state suitable for organoid formation was to grow them in a dish with mouse skin cells, referred to as fibroblast feeders, since these provide essential chemical signals and structural support that helps stem cells expand and preserve their immaturity over time.
Unfortunately, they also discovered that using mouse cells could make organoids less suitable for the development of cellular therapies to replace diseased or damaged neural tissues. Further, these feeder-supported methods are more laborious than the stem cell growth methods many labs commonly use.
The team next turned to RNA sequencing and computational analysis to pinpoint genetic differences between stem cells that produce good organoids and those that do not. This enabled them to identify four molecules — all belonging to the transforming growth factor beta superfamily of molecules — that were responsible for keeping stem cells in a less-developed state.
Adding these four molecules to stem cells growing in a dish kept them in an immature state and enabled these cells to produce high-quality, well-structured organoids.
“We have taken mouse cells out of the equation while retaining some of their benefits for organoid formation, bringing us closer to our goals of studying and developing treatments for complex neurological diseases,” concluded Bennett Novitch, the senior author of the paper.
Related topics
Disease Research, Neurosciences, Organoids
Related conditions
Alzheimer’s disease, autism spectrum disorder (ASD)
Related organisations
UCLA
Related people
Assistant Professor Momoko Watanabe, Professor Bennett Novitch