China approves IND application for Everest Medicines novel BTK inhibitor
Posted: 18 October 2022 | Victoria Rees (Drug Target Review) | No comments yet
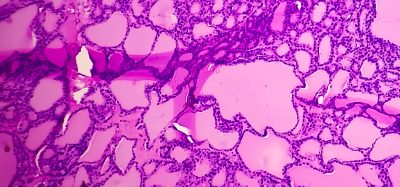
The Chinese National Medical Products Administration has approved a Phase Ib study of Everest Medicines’ EVER001, for potential treatment of glomerular diseases.

Everest Medicines has announced that the China National Medical Products Administration’s (NMPA) Center for Drug Evaluation has approved the Investigational New Drug (IND) application for a Phase Ib study of EVER001 (previously known as XNW1011). EVER001 is a next-generation covalent reversible Bruton’s tyrosine kinase (BTK) inhibitor, in development for the treatment of glomerular diseases.
BTK is an essential component of the B-cell receptor signalling pathways that regulate the survival, activation, proliferation and differentiation of B lymphocytes. Targeting BTK with small molecule inhibitors has been demonstrated to be an effective treatment option for B-cell lymphomas and autoimmune diseases.
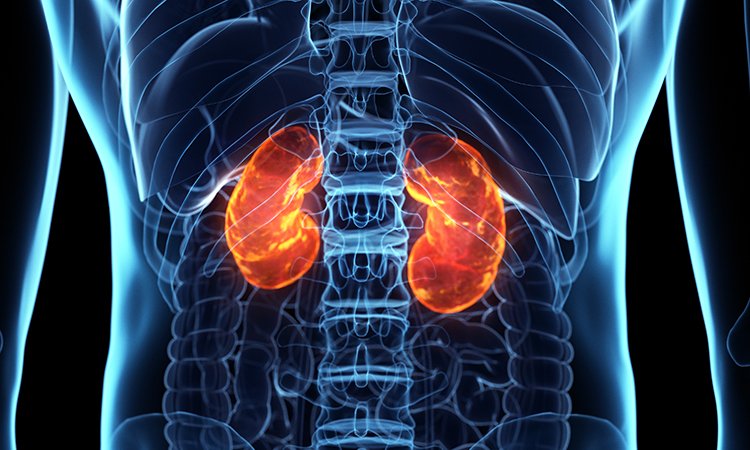
The planned Phase Ib clinical study will evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of EVER001 in patients in China with glomerular disease characterised by proteinuria, a common cause of chronic kidney disease. Under an exclusive licensing agreement with Sinovent Pharmaceuticals and SinoMab BioScience in September 2021, Everest owns the global rights to develop, produce and commercialise EVER001 for the treatment of renal diseases. Based on a Phase I study in healthy subjects in China conducted by SinoMab, EVER001 exhibited high selectivity, excellent pharmacokinetics properties and safety profile as well as robust target engagement.
“The advancement in clinical development of EVER001 for glomerular diseases represents one of the potentially first-in-class therapeutic opportunities that exist across Everest’s broad pipeline and highlights our long-term growth strategy to advance innovative and high-quality therapies for the benefit of patients worldwide with unmet demand,” said Dr Zhengying Zhu, Chief Medical Officer for Internal Medicine at Everest Medicines. “Chronic kidney disease is a leading global public health problem and will remain as a top area of therapeutic focus for Everest Medicines. The initiation of this Phase Ib trial, alongside the pivotal-stage development of Nefecon, our lead asset in renal disease, strengthens the company’s commitment in this space and we look forward to progressing these important potential therapies as quickly as possible.”
Related topics
Clinical Trials, Drug Development, Legal & Compliance, Pharmacology, Small Molecules, Therapeutics
Related conditions
B cell lymphoma, Chronic kidney disease, Glomerular disease
Related organisations
China National Medical Products Administration (NMPA), Everest Medicines
Related people
Dr Zhengying Zhu