‘Chemical cube’ tools for building new drugs
Posted: 29 March 2023 | Ria Kakkad (Drug Target Review) | No comments yet
In an ongoing discovery programme, a chemical-creation platform is being revealed – based on cubic molecules – that could help breathe new life into tired drugs.
Researchers from the University of Queensland (UQ) and the Commonwealth Scientific and Industrial Research Organisation (CSIRO), both Australia, have been developing a chemical-creation platform – based on cubic molecules – that could help breathe new life into tired drugs. Details of the programme were recently published in Chemical Science.
Professor Craig Williams from UQ said the platform, developed in collaboration with CSIRO, is developing an exciting set of tools for chemists who, in many cases, had been running out of new chemical building block options.
“Chemists have often relied on hydrocarbons, substances like petrochemicals, throughout history to build new critically important chemicals for society,” Professor Williams said.
“But one key hydrocarbon that has been historically missing from this mix is cubane – a synthetic hydrocarbon in the shape of a cube,” he continued. “Cubane traditionally was overlooked, as there was no way to synthesise this molecule on a large scale and so its application was limited.
“This has since changed as Australian chemists at CSIRO reported a kilogram scale synthesis, which is now in production at Boron Molecular in Melbourne, that enabled a significant upsurge of cubane research in the twenty-first century.”
For the first time, the team at UQ successfully incorporated a nitrogen atom into a close relative of cubane, which has the long-term potential of improving the properties of this class of molecules for use in biological systems.
“Hydrocarbons find success within a wide selection of drugs, but the nature of an all-carbon atom core can impede some biological interactions and restrict their application in drug discovery,” Professor Williams said.
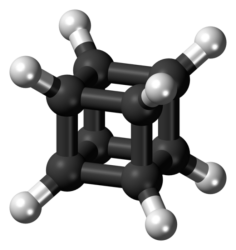
The cubane molecule before UQ researchers added nitrogen, transforming it into 1-Azahomocubane
[Credit: UQ].
“Elements like nitrogen can facilitate biologically desirable interactions that are unavailable to hydrocarbons.
“In fact, the biological demand for nitrogen is so great that most US Food and Drug Administration-approved drugs contain at least one nitrogen atom.
“Substituting nitrogen atoms into pharmaceutically proven hydrocarbon scaffolds, like cubane, is an underutilised but attractive strategy to upgrade their biological potential.
“The synthesis and study of 1-azahomocubane in collaboration with the University of Chicago, US, and Queensland University of Technology, Australia, pushes the boundary of what is possible.”
“This groundwork may lead to better treatments for disease, or day-to-day chemicals that vastly improve our quality of life and the environment,” Dr Savage said.
“To be clear, these are all future aspirations – and could be a long way off – but this work is fundamental to providing new options to chemists around the globe, and we are thrilled to have been able to make a contribution towards this goal.”
Related topics
Drug Development, Drug Repurposing, Molecular Biology
Related organisations
Commonwealth Scientific and Industrial Research Organisation (CSIRO), Queensland University of Technology (QUT), University of Chicago, University of Queensland (UQ), US Food and Drug Administration (FDA)
Related people
Professor Craig Williams