Rare genetic variant blocks common Alzheimer’s disease risk factor
Posted: 14 November 2023 | Drug Target Review | No comments yet
New understanding of “Christchurch mutation” in the APOE gene may lead to novel Alzheimer’s disease treatments.
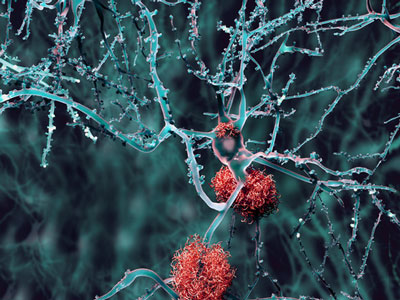
A rare genetic variant called the “Christchurch mutation” has been found to block harmful effects of apolipoprotein E4, the best-established risk factor for the most common form of Alzheimer’s disease, by scientists at the Gladstone Institutes.
It has long been known that the apolipoprotein E (APOE) gene affects the risk of Alzheimer’s disease through its three main variants: E2 (low risk), E3 (intermediate risk), and E4 (high risk). Recently, a genetic change in this gene, the “Christchurch mutation,” was discovered in a woman who did not develop Alzheimer’s disease, despite having inherited the PSEN1 gene that causes an extremely rare but highly aggressive form of the disease. This indicated that the Christchurch mutation could protect against Alzheimer’s disease.
Now, Gladstone Institutes researchers discovered that engineering the Christchurch mutation into the APOE4 gene reduces the APOE4-dependent accumulation of the protein tau, neuroinflammation, and neurodegeneration in models of Alzheimer’s disease.
Dr Yadong Huang, senior author of the study, said: “It seemed likely that this woman was protected from early-onset Alzheimer’s disease because of the APOE Christchurch mutation.” Huang is a Director of the Center for Translational Advancement at Gladstone, and Professor of Neurology and Pathology at UC San Francisco. “We immediately wondered whether this mutation could also be protective against late-onset Alzheimer’s, which accounts for the vast majority of cases of the disease.”
“It’s really exciting that the Christchurch mutation can lead to such broad protection.”
“It’s really exciting that the Christchurch mutation can lead to such broad protection.” He continued: “It opens the door to novel therapeutic interventions that could mimic the beneficial effects of this mutation.”
CRISPR
Dr Huang’s lab had already been studying the impact of APOE4 on brain cells. They had developed strains of mice, whose own APOE genes were replaced with human APOE genes, and that also produce the human tau protein, which accumulates in the brain with aging and in Alzheimer’s disease.
Dr Maxine Nelson, a former graduate student in Huang’s lab and the paper’s lead author, and her collaborators, further engineered those strains of mice to also have the Christchurch mutation. Using CRISPR gene editing, they altered the APOE4 gene in human induced pluripotent stem cells generated from an Alzheimer’s disease patient’s blood cells, which then developed into mature neurons in a dish.
Molecular mechanisms
Mice carrying human APOE4 and tau genes but not the Christchurch mutation had lots of the expected signs of Alzheimer’s disease. The tau protein accumulated in neurons, neuroinflammation levels increased in the brain, and disease-associated microglia became more widespread.
However, introducing the Christchurch mutation into these mouse models prevented or drastically reduced these abnormalities.
The scientists identified molecular mechanisms that may contribute to positive effects of the Christchurch mutation in the brain by comparing human neurons that produced APOE4 with or without the Christchurch mutation in cell culture. These mechanisms relate to the movement of tau into neurons, which might contribute to neuronal impairments in Alzheimer’s disease. The uptake of tau is mediated by cell surface molecules named heparan sulphate proteoglycans (HSPGs) and is greatly affected by APOE and the Christchurch mutation.
“The results suggest that blocking the interaction of APOE4 with HSPGs could help treat or prevent Alzheimer’s disease in people with the APOE4 gene,”
Gladstone Investigator and co-author of the new study Dr Robert Mahley showed APOE’s interaction with HSPGs 30 years ago. It was discovered in the new study that the Christchurch mutation strongly reduces the ability of APOE4 to bind HSPGs. This effect leads to a notable reduction in neuronal tau uptake.
“The results suggest that blocking the interaction of APOE4 with HSPGs could help treat or prevent Alzheimer’s disease in people with the APOE4 gene,” Dr Huang said. “This might be accomplished with small molecule drugs, monoclonal antibodies, or gene therapy. However, more work is needed before such treatments can be developed and tested.”
The study was published in Nature Neuroscience.
Related topics
Drug Targets, Gene Therapy, Monoclonal Antibody, Small Molecules
Related conditions
Alzheimer's disease (AD)
Related organisations
Gladstone Institutes