New enzyme tech offers hope for mitochondrial disease treatment
Posted: 13 May 2025 | Drug Target Review | No comments yet
Japanese researchers have developed a new enzyme technology that can precisely alter the levels of mutated mitochondrial DNA in patient-derived stem cells, offering a promising new approach for treating mitochondrial disorders.

Mitochondrial diseases impact around 1 in 5,000 people worldwide, and are notoriously difficult to treat due to the complex nature of mitochondrial DNA (mtDNA). Many of these conditions, including mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes syndrome (MELAS) and mitochondrial diabetes, are linked to mutations like m.3243A>G, a common mutation that occurs in the MT-TL1 gene, yet the tools for manipulating mtDNA have been limited.
One of the key obstacles in treating mitochondrial diseases is heteroplasmy, this is the presence of both normal and mutated mtDNA within the same cell. The ratio between the two can vary widely across different tissues, making both diagnosis and treatment efforts complicated. The ability to study how mutation load impacts disease progression has been hampered by the lack of cellular models with precisely controlled mtDNA ratios. Without these models, researchers cannot fully understand how different levels of mutated mtDNA contribute to disease symptoms.
To combat this, a research team in Japan has developed an enzyme-based technology that allows scientists to selectively increase or decrease specific mtDNA mutations in patient-derived stem cells. This advancement will allow for new possibilities both in disease modelling and therapy development.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
A new tool for precise mtDNA manipulation
The team, led by Senior Assistant Professor Naoki Yahata of Fujita Health University, engineered a class of enzymes called mitochondrial-targeted platinum transcription activator-like effector nucleases (mpTALENs). These specialised enzymes are capable of selectively targeting and cutting either mutant or normal mtDNA.
The study, published in Molecular Therapy Nucleic Acids, demonstrated that this technology can shift mutation loads in either direction. Using induced pluripotent stem cells (iPSCs) derived from patients with the m.3243A>G mutation, researchers achieved mutation loads ranging from 11 percent to 97 percent, which is a first in mitochondrial research.
Key innovations and enhancements
The success of the mpTALENs is due in part to several technical enhancements:
- Novel repeat-variable di-residues (RVDs): improved the specificity of the enzymes.
- Obligate heterodimeric fokI domains: minimised off-target effects and unwanted DNA degradation.
- Uridine supplementation: helped maintain the viability of cell lines, even those with high mutation loads that typically grow poorly.
These innovations ensured that the modified cells retained their ability to differentiate into various tissue types, making them ideal for disease modelling.
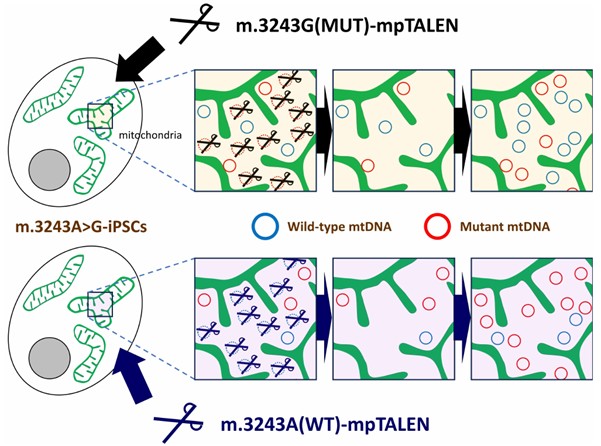

Altering levels of mutant mitochondrial DNA- The proposed system can selectively increase or decrease the proportion of mutant to normal (or ‘wild-type,’ WT) mitochondrial DNA, serving as a fundamental tool in mitochondrial disease research and paving the way to novel therapeutic strategies. Credit: Dr Naoki Yahata from Fujita Health University School of Medicine, Japan
From research model to potential therapy
This method provides scientists with isogenic cell lines, which are genetically identical, except for their mtDNA mutation load. These models are invaluable for studying how specific mutation percentages correlate with disease severity.
More importantly, the technology shows promise as a therapeutic approach. By selectively reducing the mutant mtDNA load in patient cells, mpTALENs could one day be used to treat mitochondrial diseases directly.
“Our proposed method could be adapted for other mutant mtDNAs and may contribute to understanding their associated pathologies and developing new treatments, potentially benefiting patients with various forms of mitochondrial disease” says Dr Yahata.
With this new tool, researchers now have a precise and flexible way to manipulate mtDNA in living cells. This not only fills a big gap in mitochondrial disease research but also lays the groundwork for future gene therapies aimed at correcting mitochondrial mutations at their source, offering new hope where few treatments currently exist.
Related topics
Disease Research, Drug Discovery Processes, Enzymes, Gene Therapy, Induced Pluripotent Stem Cells (iPSCs), Mitochondria, Translational Science
Related conditions
Mitochondrial disease
Related organisations
Fujita Health University