Vascularised stem cell organoids advance diabetes therapy
Posted: 28 May 2025 | Drug Target Review | No comments yet
A team of researchers have developed the first vascularised organoid model of human pancreatic islets, which could lead to further development of advanced cell therapies for diabetes.


In a major step forward for diabetes research, an international team led by Professor Maike Sander, Scientific Director of the Max Delbrück Center, has developed the first vascularised organoid model of human pancreatic islets derived from pluripotent stem cells. The study, published in Developmental Cell, showcases the advanced model’s ability to more accurately mimic the natural environment of the pancreas, opening up new possibilities for the study of diabetes and the development of cell-based therapies.
The importance of pancreatic islets in diabetes
Pancreatic islets are clusters of hormone-secreting cells within the pancreas, including insulin-producing beta cells that regulate blood sugar levels. In individuals with diabetes, particularly Type 1 diabetes, these cells are damaged or destroyed, leading to impaired insulin production. For years, scientists have relied on stem cell-derived islet organoids (SC-islets) to study pancreatic function and disease. However, a critical limitation has been the immaturity of beta cells in these models, which fail to replicate the complex interactions of real islets in the body.
Sander’s team overcame this challenge by engineering SC-islet organoids with integrated blood vessels. By combining human endothelial cells (which form blood vessels) and fibroblasts (which help create connective tissue), they successfully created organoids where tube-like vessels grew into and around the SC-islets. This vascular network provided essential signals and structural support, allowing beta cells to mature and function more like their natural counterparts.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
“Our results highlight the importance of a vascular network in supporting pancreatic islet cell function,” says Sander. “This model brings us closer to replicating the natural environment of the pancreas, which is essential for studying diabetes and developing new treatments.”
A significant impact on beta cell maturity
Sander credits five years of rigorous experimentation by a dedicated team of stem cell biologists and bioengineers, who tested various combinations of cell types and culture conditions before identifying the right “recipe” to support vascularised islet development.
The impact of vascularisation was strong; as the organoids secreted significantly more insulin in response to glucose than their non-vascularised counterparts, indicating a higher proportion of mature beta cells.
Further investigation revealed two key mechanisms behind this maturation:
- Endothelial cells and fibroblasts help build the extracellular matrix– a web of proteins and carbohydrates essential for cell growth.
- Endothelial cells also secrete bone morphogenetic protein (BMP)– a molecule that stimulates beta cell development.
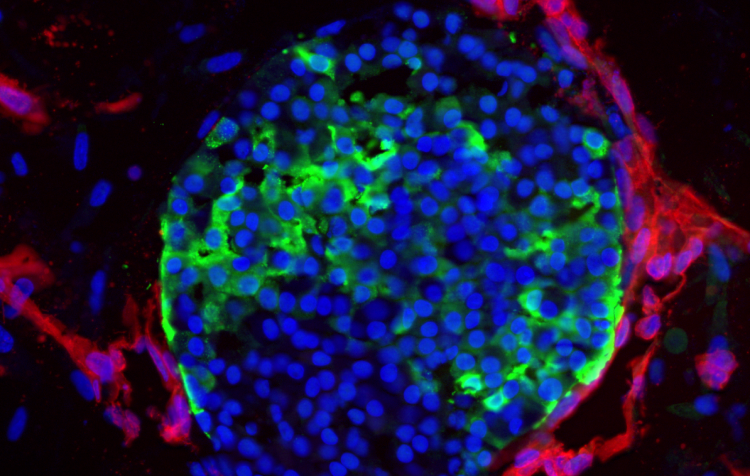

Researchers have devised the right conditions to grow vascularized stem cell islets. The image shows vasculature (red) tightly wrapped around insulin-producing cells (green) in the islets (blue). Image credit: Sander lab
Enhancing the model with microfluidics
To further improve physiological realism, the team integrated the vascularised organoids into microfluidic devices- tiny chips that mimic blood flow by pumping nutrient-rich fluid through the vascular networks. This additional layer of complexity further boosted beta cell maturation.
“We found a gradient,” explains Sander. “Non-vascularised organoids had the most immature cells, a greater proportion matured with vascularisation, and even more matured when we introduced nutrient flow through blood vessels.”
Real-world potential: success in preclinical models
The final test came in diabetic mice. Mice grafted with vascularised SC-islets producing more insulin and had better outcomes than those receiving non-vascularised grafts. Some mice showed no signs of diabetes 19 weeks after transplantation, underscoring the promise of vascularised SC-islets for future cell therapies.
Toward a better model for type 1 diabetes
Sander’s team now plans to use vascularised SC-islet organoid models to study Type 1 diabetes, where the body’s immune system attacks and destroys beta cells. By creating vascularised organoids from Type 1 diabetes patients and introducing their immune cells into the system, the researchers hope to uncover how immune cells target beta cells and explore new strategies for preventing or reversing this autoimmune attack.
“Our approach provides a more realistic model of islet cell function and could help develop better treatments in the future,” says Sander.
This breakthrough could lead to more accurate disease modelling, better understanding of diabetes pathogenesis, and the development of advanced cell therapies-offering renewed hope to the millions living with diabetes worldwide.
Related topics
Animal Models, Bioengineering, Cell Therapy, Cell-based assays, Disease Research, Drug Discovery Processes, Immunology, Organoids, Regenerative Medicine, Stem Cells, Translational Science
Related conditions
type 1 diabetes
Related organisations
Max Delbrück Center