Stem cell liver model reproduces rare immune drug reactions
Posted: 17 October 2025 | Drug Target Review
A new human liver organoid platform could help predict which drugs trigger dangerous immune reactions in susceptible patients.

Researchers at Cincinnati Children’s Hospital Medical Center, in collaboration with Roche, have developed a next-generation human liver organoid microarray platform. The platform is designed to predict which drugs might trigger harmful immune responses in some patients, offering a potential for safer, more personalised drug development.
The study outlines a miniaturised, fully human liver system created from stem cells and a patient’s own immune cells. The system offers a powerful new tool to understand why certain medications can cause serious, immune-related liver injuries.
“Our goal was to create a human system that captures how the liver and immune system interact in patients,” says co-first author Dr Fadoua El Abdellaoui Soussi. “By integrating patient-specific genetics and immune responses, we can finally begin to explain why certain drugs cause liver injury in only a small subset of individuals.”
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
A human model of immune-driven liver injury
Some drugs that pass early safety testing can still cause idiosyncratic drug-induced liver injury (iDILI), a rare immune reaction that can lead to severe illness or even drug withdrawal. Standard laboratory tests and animal models cannot replicate these complex, patient-specific immune mechanisms.
Some drugs that pass early safety testing can still cause idiosyncratic drug-induced liver injury (iDILI), a rare immune reaction that can lead to severe illness or even drug withdrawal.
The new platform addresses this gap by combining induced pluripotent stem cell (iPSC)-derived liver organoids with each donor’s autologous CD8⁺ T cells – the immune cells responsible for attacking infected or damaged tissue. In combination, they form a human, immune-competent system that reproduces the genetic and immune variation found in patients.
To prove the concept, the researchers then recreated liver injury caused by the antibiotic flucloxacillin, which affects only carriers of the HLA-B*57:01 risk gene. The model reproduced classic signs of immune-mediated liver toxicity, including T cell activation, cytokine secretion and hepatocyte damage, closely matching the responses observed in susceptible patients.
“Our goal has always been to bring human biology into the lab in a way that’s scalable, reproducible, and meaningful for patients,” says corresponding author Dr Magdalena Kasendra, who is also director of research and development at CuSTOM. “By linking foundational stem cell science with applied toxicology, this model moves organoid research another step closer to transforming how drugs are developed and tested.”
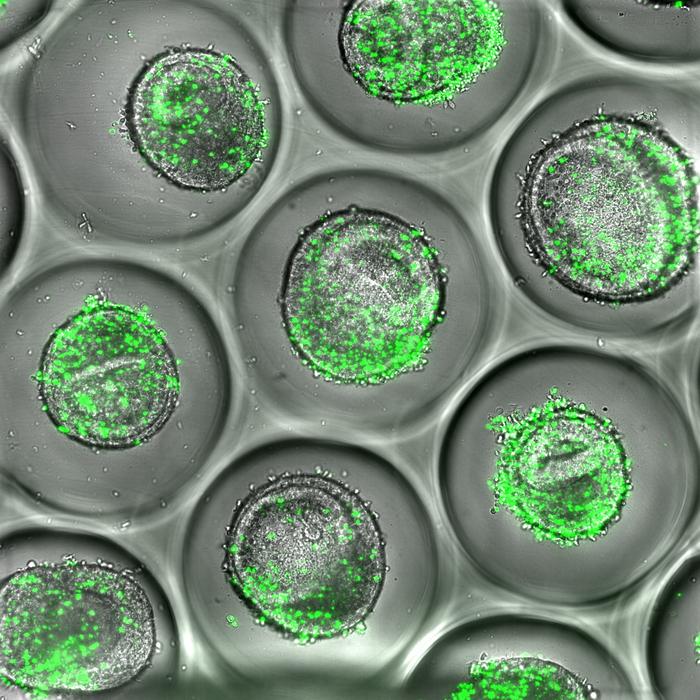

Example of human liver organoids co-cultured with autologous CD8 T cells (green). These tissues can be used during drug development to predict liver toxicity, according to new resaerch published by experts at Cincinnati Children’s. Credit: Cincinnati Children’s
Building on prior research
The platform builds on previous work by study co-author Dr Takanori Takebe, MD, whose lab pioneered reliable methods for creating human liver organoids from iPSCs. By adapting these techniques into a matrix-free microarray format and integrating patient-matched immune cells, the CuSTOM Accelerator team has transformed this innovation into a scalable, precision toxicology system.
“This partnership shows the power of combining academic innovation with industry experience,” says Dr Adrian Roth, principal scientific director of Personalised Healthcare Safety at Roche. “Together we’re building predictive human models that can improve patient safety and accelerate the development of new medicines.”
A growing ecosystem for organoid medicine
Looking ahead, the team is working to automate organoid assays and enable high-throughput screening across large, genetically diverse donor populations. This next phase aims to capture the full spectrum of human variability – which could potentially lead to the development of therapies that are more effective, inclusive and personalised.
“This work reflects the vision of CuSTOM – to turn human organoid science into practical tools that improve health,” Kasendra says. “This is just the beginning – by bridging biology, engineering, and clinical insight, we’re getting closer to predicting how real patients will respond to new treatments before they ever reach the clinic.”
Related topics
Drug Development, Drug Discovery, Immunology, In Vitro, Induced Pluripotent Stem Cells (iPSCs), Organoids, Precision Medicine, Stem Cells, T cells, Toxicology, Translational Science
Related conditions
Immune-related liver injuries
Related organisations
Cincinnati Children's Hospital Medical Center