TRF1 protein identified as potential obesity drug target
Posted: 28 October 2025 | Drug Target Review | No comments yet
Researchers have discovered that removing a telomere-protecting protein, TRF1, makes mice leaner and metabolically healthier without shortening their telomeres, potentially leading to new methods for tackling obesity and age-related diseases.

A new study from Spanish National Cancer Centre (CNIO) has revealed that depletion of the TRF1 telomere-binding protein leads to leaner mice with healthier metabolic profiles.
Exploring TRF1 beyond telomere protection
TRF1 is best known for its role in safeguarding telomeres – the protective caps at the ends of chromosomes. However, the CNIO research team discovered that the protein also plays a previously unknown part in controlling body metabolism.
The study’s findings indicate that TRF1 affects metabolic health through pathways separate from its established role in maintaining telomeres.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
To investigate this connection, scientists compared normal mice with genetically modified ones that lacked TRF1. Mice without the protein stayed leaner over time, resisted fat accumulation and exhibited healthier blood sugar and insulin levels than normal mice. These advantages occurred without any detectable shrinking of telomeres.
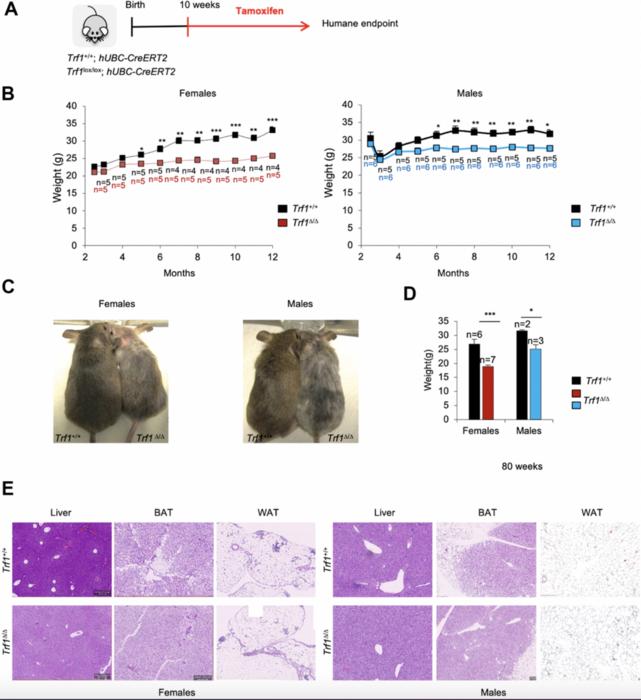

Depletion of Trf1 in mice induces weight loss. (A) Experimental plan: Trf1+/+ or Trf1lox/lox; hUBC-CreERT2 mice start to receive tamoxifen treatment intraperitoneally at 10 weeks of age until humane endpoint. (B) Weight follow-up in females (left) and males (right) in both genotypes. Note that TrfΔ/Δ females start to weigh less than wild-type at five months and males at six months. (C) Representative images of wild-type and Trf1 deleted mice of 10 months of age. Note the observable difference in weight and graying hair in Trf1Δ/Δ mice. (D) Measurement of body weight in 80-week-old mice. Note that the difference in weight is maintained throughout their lifespan. (E) Hematoxylin and eosin staining of liver, white and brown adipose tissue. Note that there are no differences between genotypes regarding liver and white adipose tissue. In brown adipose tissue, Trf1Δ/Δ mice present fewer and smaller lipid droplets than wild-type mice. Error bars, s.e.m.; only significant values are shown; *P < 0.05; **P < 0.01; ***P < 0.001 determined by two-tailed Student’s t-test (B, D). Credit: 2025 Louzame et al
Energy processing, not activity, drives fat loss
The researchers noted that the leaner body composition in TRF1-deficient mice did not stem from eating less or being more active. Instead, this fat loss came from biological changes in how energy was processed and stored.
Male mice without TRF1 gained less weight and had lower LDL cholesterol levels even when fed a high-fat diet. Female mice displayed milder effects, a difference consistent with known sex-based variations in susceptibility to diet-induced obesity.
Shifts in liver gene activity and broader implications
Analysis of liver gene expression demonstrated changes in multiple key pathways. Genes involved in fat production, energy metabolism and muscle growth were downregulated, while those associated with inflammation and cholesterol synthesis were upregulated. The mice also exhibited increased energy expenditure and appeared to shift from using fat to protein as an energy source, likely due to their reduced fat stores. However, some older mice showed signs of mild liver stress, including fibrosis and DNA damage, indicating a potential long-term trade-off.
This study widens our understanding of telomere-related proteins, showing how they impact more than just cellular aging.
Overall, this study widens our understanding of telomere-related proteins, showing how they impact more than just cellular aging. By revealing a link between TRF1 and metabolism, the findings highlight new opportunities to target TRF1 or its pathways for treating obesity and related metabolic disorders. Further research is needed to determine exactly how TRF1 regulates fat development and whether these effects translate to humans.
The findings provide a foundation for future investigations into how telomere-binding proteins could provide potential therapeutic targets for metabolic and age-related diseases.
Related topics
Animal Models, Drug Discovery, Drug Discovery Processes, Metabolomics, Molecular Biology, Obesity, Protein, Therapeutics, Translational Science
Related conditions
Age-related diseases, Obesity
Related organisations
Spanish National Cancer Centre (CNIO)