Tau protein disrupts molecular transport in neurons in Alzheimer’s
Posted: 5 September 2018 | Iqra Farooq (Drug Target Review) | No comments yet
Researchers have discovered how the tau protein associated with Alzheimer’s affects molecular transport and a nucleporin called Nup98…
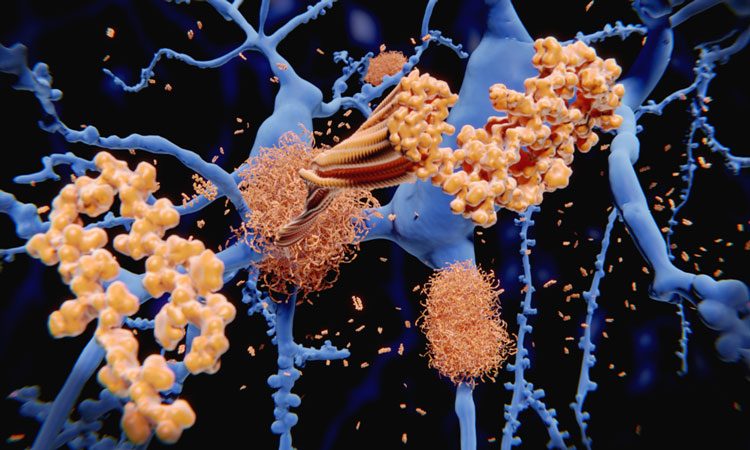

Researchers from multiple institutions and led by those at Massachusetts General Hospital and Johns Hopkins School of Medicine found how tau proteins that accumulate in the neurofibrillary tangles in Alzheimer’s disease disrupt the ordinary function of the brain cells.
The team described how tau interferes with communication between the nucleus of the neurons and the rest of the cell body.
“Communication between the nucleus and the rest of the cell is usually a tightly regulated process,” said one of the senior authors of the study, Dr Bradley Hyman, who is also the director of the MGH-based Massachusetts Alzheimer’s Disease Research Centre.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
“Our work shows a new way tau might cause brain cells to become impaired. In other systems, disruption of this communication causes cell misfunction and even cell death, so we think this might contribute to neuronal dysfunction and death in Alzheimer’s disease as well.”
The team worked on experiments in neurons from patients with Alzheimer’s disease, and used cellular models of tau-based neuropathology. They found that the Alzheimer’s-associated tau interacts with a nucleoporin called Nup98.
The interaction of tau and Nup98 led to Nup98 being mislocated to the cytoplasm, where it promoted the aggregation of tau into neurofibrillary tangles
They also found that the nuclei of neurons from Alzheimer’s patients took up large test molecules, indicating that the nuclear pore complexes had become leaky. The structures were also reduced in number and unevenly distributed throughout the nuclear membrane.
Neurons from mice genetically programmed to develop tau brain tangles also showed similar nuclear pore complex leakage, allowing passage of large dye molecules into the nuclei.
The level of Ran enzymes depleted from the animals’ neuronal nuclei, and changes in shape and structure were identified by the team. By suppressing the expression of the abnormal tau gene, the researchers restored nuclear levels of Ran and levels of Nup98 in the nuclear membrane.
By decreasing levels of Nup98 in neurons from the mice, the team restored an appropriate Ran ratio between the nucleus and cytoplasm, showing that tau was the cause of the Ran abnormalities.
“One of the exciting things about these findings is that, if we can block the interaction between tau and the nuclear pore, it might allow existing neurons to become more functional in patients; so one of our next steps will be determining whether or not that is possible” says Dr Hyman. “The collaboration between groups from across the country – including Johns Hopkins, the Mayo Clinic and the Salk Institute – with each group adding its special expertise, made this work possible.”
The study was published in the journal Neuron.
Related topics
Disease Research, Drug Discovery Processes, Drug Targets, Neurons, Research & Development, Therapeutics
Related conditions
Alzheimer’s disease
Related organisations
Johns Hopkins School of Medicine, Massachusetts General Hospital
Related people
Dr Bradley Hyman