Macrophages identified as drug target for serous cavity cancers
Posted: 6 April 2020 | Victoria Rees (Drug Target Review) | No comments yet
The loss of RXR function, a molecular mechanism which determines the identity macrophages, has been revealed as a drug target for cancers in serous cavities.

A new molecular mechanism has been discovered, mediated by nuclear receptors, which determines the identity and expansion of macrophages. The newly discovered mechanism specifically affects the macrophages resident in the serous cavities, the membrane-surrounded cavities that enclose and protect many organs. According to the researchers, the findings could have important implications for the treatment of diseases that affect the serous cavities and the organs they contain, including many cancers and myocardial infarction.
The study was conducted at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), Spain, in collaboration with researchers at Mount Sinai Hospital, US.
“Macrophages are immune-system cells whose main function is to act as sentinels in the body, defending it against pathogens. Macrophages are also important for maintaining homeostasis – the balanced and stable functioning of the body’s systems – by eliminating tissue waste products and debris from tissue repair,” explained Dr Mercedes Ricote, study co-ordinator and head of the Nuclear Receptor Signaling group at the CNIC. “In recent years, work from many groups has changed the understanding of the hemopoietic origin and regulation of macrophages. These studies have revealed enormous heterogeneity among tissue macrophages, depending on their embryonic or hemopoietic origin and on the miroenvironmental signals that modulate their identity and maintenance in adult life.”
There are three serous membranes: the peritoneum, which covers the abdominal cavity; the pleura, surrounding the lungs; and the pericardium, which covers the heart. “One of the main functions of the macrophages residing in these cavities is to maintain homeostasis by removing dead cells,” explained Ricote. In addition, recent studies have demonstrated that these macrophages can infiltrate adjacent injured organs, “generating an effective rapid repair response that is independent of the recruitment of macrophage precursors via the blood supply.”
The researchers explain that the expansion of peritoneal macrophages after birth and their maintenance during adult life are controlled by retinoid X receptor (RXR), a member of the nuclear receptor family.
“RXR exists in the interior of cells,” explained joint first author Dr Mª Piedad Menéndez at CNIC, “where it detects the presence of lipids and derivatives of vitamin A. These ligands activate RXR, triggering the expression of specific genes that control development, immunity, homeostasis and metabolism.”
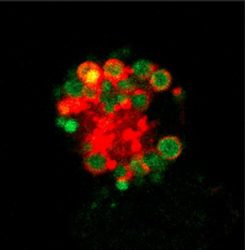

Confocal microscopy image showing a peritoneal macrophage from a mouse lacking RXR. The image shows a high lipid content (green) in acid vesicles (red) [credit: CNIC].
The research team showed that RXR is required to establish a population of macrophages in the serous cavities of newborn mice by controlling their proliferation, as well as being important for the survival of these macrophages in adults. Menéndez explained: “The absence of RXR from serous-cavity macrophages results in a massive accumulation of toxic lipids that kills the cells through a process called apoptosis.”
Using models of ovarian cancer in mice, the researchers showed that peritoneal macrophages can infiltrate ovarian tumours and act as ‘tumour-associated macrophages’ that support tumour growth.
Therefore, the loss of RXR function leads to a decrease in the number of macrophages in the peritoneal cavity, resulting in a decreased contribution of these macrophages to growing ovarian tumours, slowing the progression of the disease.
“Serous cavity macrophages thus represent a possible target for the treatment of cancers that affect organs in the serous cavities,” said Ricote.
The results were published in Nature Communications.
Related topics
Drug Targets, Immunology, Oncology, Research & Development, Targets
Related conditions
Cancer
Related organisations
Centro Nacional de Investigaciones Cardiovasculares (CNIC), Mount Sinai Hospital
Related people
Dr Mercedes Ricote, Dr Mª Piedad Menéndez