Multivalent carbohydrate-binding modules show efficacy against COVID-19
Posted: 8 May 2020 | Victoria Rees (Drug Target Review) | No comments yet
Tested on plaque reducing assays, researchers have identified a lead candidate multivalent carbohydrate-binding module for the treatment of COVID-19.
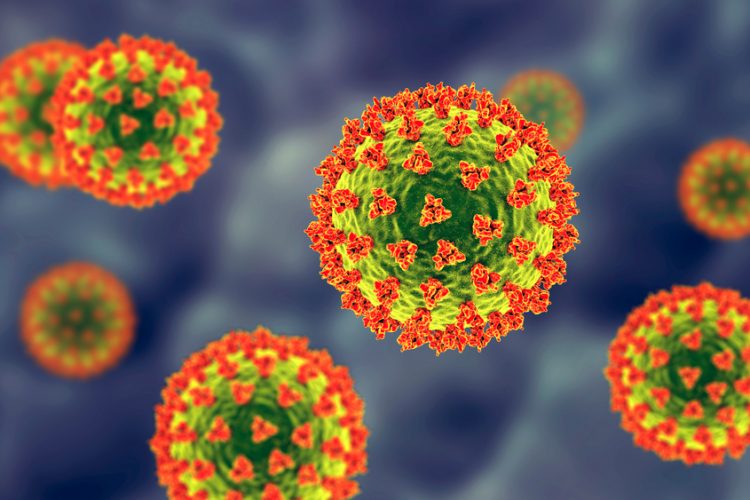

A University of St Andrews, UK, spinout company, focused on treating infectious disease and oncology by targeting the human glycome, has announced results from three separate in vitro studies into preventing coronavirus infections including SARS-CoV-2, the cause of the COVID-19 pandemic.
Pneumagen Ltd used its lead candidate and other first-in-class multivalent carbohydrate-binding modules (mCBMs), generated using its proprietary GlycoTarge™ platform to reduce SARS-CoV-2 plaques on assays.
Working with Public Health England’s Porton facility and separately with the University of Glasgow’s MRC Centre for Virus Research, Pneumagen has tested the activity of its mCBMs against coronaviruses using plaque reduction assays. At Porton and the University of Glasgow, the company’s mCBMs were found to reduce the number of SARS-CoV-2 plaques in these assays when the mCBMs were used in both prevention and treatment of infection.
The company says these findings build on its other work, with a different clinically relevant coronavirus that can cause the common cold where a plaque reduction assay also demonstrated antiviral activity for mCBMs.
Pneumagen’s lead mCBM, Neumifil™, is already being developed for the universal treatment of respiratory tract infections (RTIs) including influenza virus (IFV) and Respiratory Syncytial Virus (RSV) and now coronaviruses. Neumifil’s novel mechanism of action, masking glycan receptors in patients’ airways thereby preventing the entry of the virus, has the potential to revolutionise the treatment of RTIs by providing clinicians with the opportunity to offer patients total protection against any circulating viral strain.
Douglas Thomson, Chief Executive Officer of Pneumagen, said: “Today’s positive results from in vitro studies of our mCBMs against coronaviruses show that glycan binding has the potential to prevent and treat infection. This further supports the value of our universal therapeutic modality to block access to lung cells of SARS-CoV-2, as well as other viruses, that causes respiratory tract infections, providing the potential for a pan-viral respiratory product. Our goal is now to rapidly begin clinical testing for the prevention and treatment of COVID-19.”
Related topics
Assays, Drug Targets, Research & Development, Target Molecule, Therapeutics
Related conditions
Coronavirus, Covid-19
Related organisations
Pneumagen Ltd, Public Health England, University of Glasgow, University of St Andrews
Related people
Douglas Thomson