Researchers reveal cryo-EM structure of remdesivir bound to SARS-CoV-2
Posted: 4 May 2020 | Victoria Rees (Drug Target Review) | 1 comment
A group of researchers has used cryo-EM to discover the structure of the remdesivir-bound RNA complex of SARS-CoV-2 and explain how the drug inhibits COVID-19 viral replication.


New research from Chinese scientists has produced the high-resolution cryo-electron microscopy (EM) structure of the remdesivir-bound RNA complex of SARS-CoV-2, the virus causing the COVID-19 pandemic.
The study was conducted by researchers at the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences (CAS), the Zhejiang University School of Medicine, Peking Union Medical College and the Chinese Academy of Medical Sciences, along with their collaborators.
SARS-CoV-2 is a positive-strand RNA virus that mainly infects human cells through the mucosal system. The massive replication of the virus requires the rapid synthesis of its genetic RNA, a process mediated by a multi-subunit replication transcription complex composed of multiple non-structural proteins (nsp) of the virus. According to the researchers, the core element for replication is the replicase complex. Remdesivir is one of several nucleoside drugs which targets replicase that is currently under clinical testing.
The scientists were able to elaborate the replicase-targeting mechanism underlying the antiviral efficacy of these nucleoside drugs. Their study reports the cryo-EM structure of the SARS-CoV-2 replicase both in the apo form at 2.8 Å resolution and in complex with a template-primer RNA and remdesivir at 2.5 Å resolution. The researchers say that the overall conformation of the complex structure is very similar to that of the apo form, with the identical structures at the core catalytic active site.
Comprehensive analysis of the structures showed that the SARS-CoV-2 replicase complex is an efficient enzyme. The team also found that during RNA extension, conformational change is small, which explains the highly contagious nature of SARS-CoV-2.
The replicase complex recognises RNA – but not DNA – through a sequence-independent binding method. The structure of the complex explains how remdesivir enters the replication active site and covalently links with the viral genome, inhibiting viral replication.
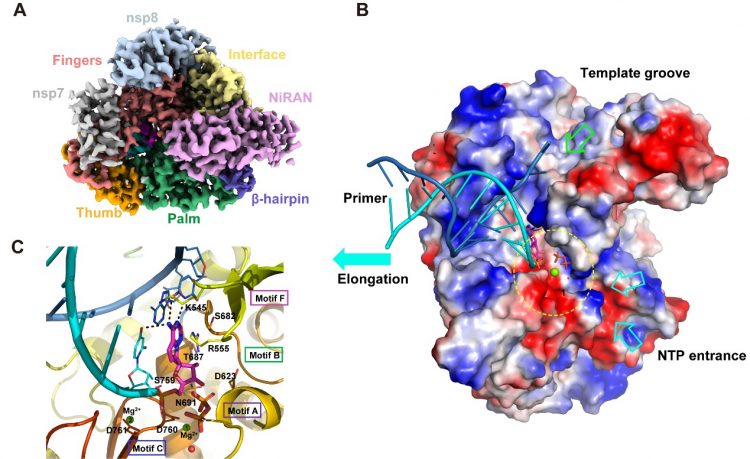

The cryo-EM structure of the RNA and remdesivir bound RdRp complex from SARS-CoV-2 [credit: SIMM].
The residues involved in RNA binding as well as those comprising the catalytic active site are highly conserved among most RNA viruses. This shows the conservative mechanism of the replicase complex during gene replication and suggests it may be possible to develop broad spectrum antiviral inhibitors.
The researchers suggest that the structures described in their study reveal potential binding patterns that offer theoretical support for the design of more powerful, efficient and specific anti-SARS-CoV-2 drugs to fight COVID-19.
The findings were published in Science.
Related topics
Drug Targets, Microscopy, Research & Development, RNAs, Structural Biology, Target Validation, Targets, Therapeutics
Related conditions
Coronavirus, Covid-19






Which cryo-EM Image processing tools were used? Thanks.