Researchers observe interaction between antibodies and target proteins
Posted: 17 August 2020 | Victoria Rees (Drug Target Review) | No comments yet
Using cryogenic electron microscopy, scientists have observed the interaction between antibodies and their target molecules, providing information that could be utilised in the development of synthetic antibodies.

Scientists report that for the first time, they have observed the interaction between therapeutic antibodies and their target protein. The research describes these molecular mechanisms and potentially opens the way for the development of new treatments.
The study was conducted by researchers at the Centre national de la recherche scientifique (CNRS), Institut Pasteur and the Université de Bordeaux, all France.
According to the researchers, non-Hodgkin lymphomas cause uncontrolled proliferation of B lymphocytes, to the detriment of healthy cells. Since the end of the 1990s, immunotherapy has been a frontline treatment using synthetic antibodies to target a protein on the surface of B lymphocytes called CD20. The body’s defences then identify these cells covered in antibodies as pathogens and destroy them.
Currently, the types of therapeutic antibodies used are classified into two groups according to how many CD20 molecules they bind to and the immunity response they trigger. Antibodies from the first group have the ability to interact with twice as many CD20 molecules than antibodies from the second group and can set off a cascading immune response called a ‘complement pathway’. Until now, the molecular mechanisms at the root of underlying the differences between these two groups were unknown.
By using cryogenic electron microscopy, the scientists were able to observe on an atomic level the interaction between the representatives of both groups of antibodies and their target molecules. They have shown that because of the availability of space, CD20 proteins can bind to two type 1 antibodies but to only one type 2 antibody. Due to their larger numbers on the surface of B lymphocytes, type 1 antibodies can form clusters. The team demonstrated that these clusters trigger the complement pathway which leads to the destruction of the target B lymphocytes. Type 2 antibodies, on the other hand, are thinly dispersed on the cell surface and therefore do not trigger a cascading response. Other independent immune responses are still able to destroy the cells.
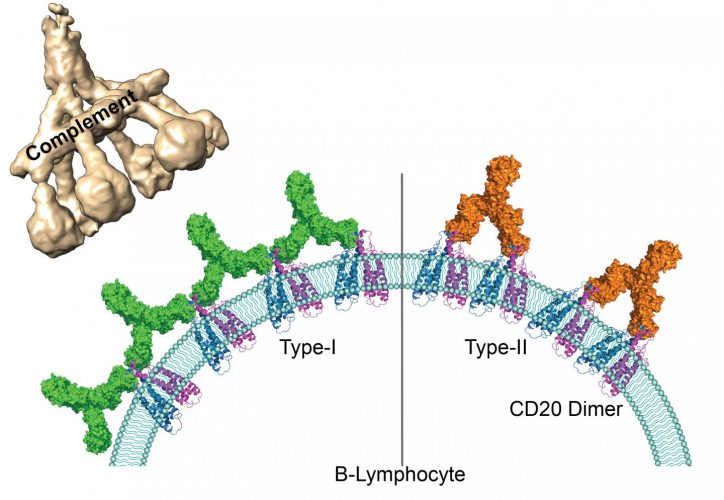

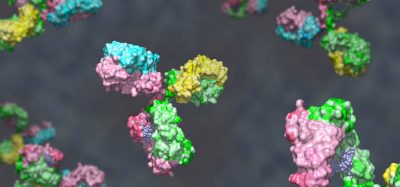
Human antigen CD20 molecules (blue and pink) expressed on the surface of B cells are recognised by type 1 (green), and type 2 (orange) therapeutic antibodies. Only type 1 antibodies can potently recruit complement C1 component (light brown) to elicit complement pathway [credit: Nicolas Reyes].
The team say this research could lead to new synthetic antibodies that are able to control a patient’s immune response. In addition, this description of the mechanism activating the complement pathway opens the way to new research possibilities aiming at an understanding of how immune defences work.
The results were published in Science.
Related topics
Antibodies, Drug Targets, Imaging, Microscopy, Protein, Proteomics
Related conditions
non-Hodgkin lymphoma
Related organisations
Centre national de la recherche scientifique (CNRS), Institut Pasteur, Université de Bordeaux