New insight to guide discovery of drugs targeting ion channels
Posted: 24 November 2020 | Hannah Balfour (Drug Target Review) | No comments yet
The study shows how drug-like small molecules inhibit the activity of Transient Receptor Potential Canonical 1/4/5 (TRPC1/4/5) channels and could transform the development of future therapies.

A new study provides insights into how xanthine-based modulators, a type of drug-like small molecules, can regulate the activity of a type of ion channels, called Transient Receptor Potential Canonical 1/4/5 (TRPC1/4/5) channels. The researchers suggest their study could transform ongoing drug development efforts targeting this channel and other related ones.
According to researchers at the University of Leeds, TRPC1/4/5 channels are formed of a heterotrimer of three proteins: short transient receptor potential channel (TRPC) 1, 4 and 5. The full heterotrimeric channel is a non-selective positive ion channel that is currently being investigated as a target for the treatment of a range of conditions, including cardiovascular disease, progressive kidney disease, depression and anxiety.
The team said that while some TRPC1/4/5 inhibitors have recently entered clinical trials, drug development had been hampered by gaps in the understanding of their mechanism of action. According to the researchers, xanthine-based modulators are the most potent and selective TRPC1/4/5 inhibitors described to date, and so they set out to show how they interact with TRPC1/4/5 channels and modulate their activity.
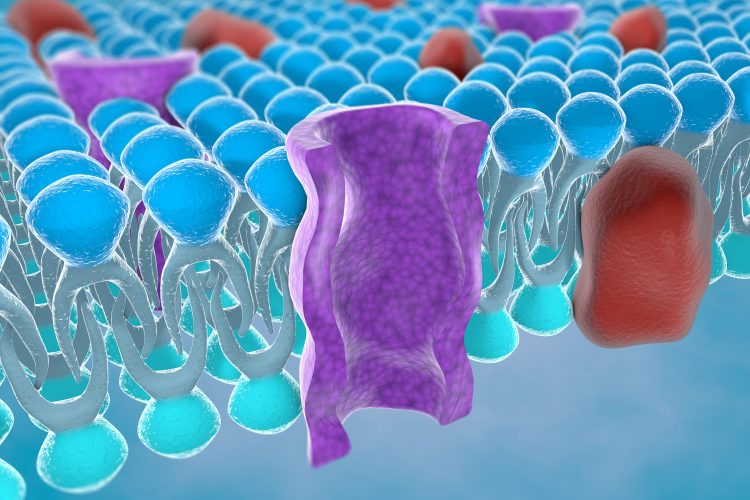
In their study published in Communications Biology, researchers led by Dr Robin Bon, Associate Professor of Chemical Biology in the School of Medicine, and Dr Stephen Muench, Associate Professor of Membrane Biology in the School of Biomedical Sciences, show how a xanthine-based selective TRPC5 inhibitor, called Pico145, binds to a conserved lipid binding site of TRPC5, where it displaces a bound phospholipid. According to the team, the binding of Pico145 to the TRPC5 protein is sufficient to prevent the TRPC1/4/5 channel opening.
![The structure of the tetrameric structure of a TRPC5 channel in complex with Pico145 (left). Each TRPC5 monomer is displayed in a different colour. The structure revealed that the drug-like small molecule Pico145 can bind to each of four pockets between subunits (middle). When the structure of TRPC5 was determined in the absence of Pico145, this pocket was occupied by a phospholipid (right) [Credit: Bon et al.].](https://www.drugtargetreview.com/wp-content/uploads/TRPC5-TRPC145-ION-CHANNEL.jpg)
![The structure of the tetrameric structure of a TRPC5 channel in complex with Pico145 (left). Each TRPC5 monomer is displayed in a different colour. The structure revealed that the drug-like small molecule Pico145 can bind to each of four pockets between subunits (middle). When the structure of TRPC5 was determined in the absence of Pico145, this pocket was occupied by a phospholipid (right) [Credit: Bon et al.].](https://www.drugtargetreview.com/wp-content/uploads/TRPC5-TRPC145-ION-CHANNEL.jpg)
The structure of the tetrameric structure of a TRPC5 channel in complex with Pico145 (left). Each TRPC5 monomer is displayed in a different colour. The structure revealed that the drug-like small molecule Pico145 can bind to each of four pockets between subunits (middle). When the structure of TRPC5 was determined in the absence of Pico145, this pocket was occupied by a phospholipid (right) [Credit: Bon et al.].
Dr David Wright, first author of the study, said: “Using cryo-electron microscopy performed in the Astbury BioStructure Laboratory, we determined high-resolution structures of the TRPC5 channel in the presence and absence of Pico145. These structures show, for the first time, how Pico145 can displace a lipid bound to each of the four TRPC5 proteins. Further studies revealed the importance of individual amino acid residues in the Pico145 binding site of TRPC5.”
Dr Bon added: “The opening and closing of TRPC channels is regulated by many factors, including dietary components such as lipids, minerals and antioxidants, as well as environmental toxins. Overactivity of TRPC channels is linked to a range of diseases. Therefore, small molecules that can stop TRPC channels from opening are increasingly considered as potential therapeutic agents.
“In Leeds, we have done a lot of work to understand how xanthines regulate TRPC channel activity. Our structures represent a break-through that may provide new, rational approaches to the development of drug candidates that target TRPC channels.
“In addition to its relevance to drug discovery, our study also provides new insights into how physiological and dietary factors such as lipids and zinc ions may regulate TRPC channels. Therefore, our work has opened up several new lines of research.”
Related topics
Analysis, Drug Targets, Imaging, Ligands, Microscopy, Proteomics, Therapeutics
Related conditions
Anxiety, Cardiovascular disease, Depression, Kidney disease
Related organisations
Leeds University
Related people
Dr David Wright, Dr Robin Bon, Dr Stephen Muench