Atomic force microscopy reveals structure of ribosome stalk proteins
Posted: 11 January 2021 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have used force atomic microscopy to show the structural dynamics at ribosome stalk proteins when building new proteins.

Researchers from Kanazawa University and Niigata University, both Japan, have used force atomic microscopy to show visualisations of the structural dynamics and factor pooling that take place at ribosome stalk proteins as they build new proteins.
According to the scientists, ribosomes are the complexes of ribonucleoproteins at the heart of protein synthesis in cells. However in the absence of conclusive evidence, how these complexes operate has been open to debate until now.
The broad function of ribosomes has been widely understood for some time – they read messenger RNA (mRNA) sequences and from that generate sequences of correctly ordered amino acids into new proteins. The ribosome stalk protein plays an integral role in the protein synthesis process by recruiting protein factors responsible for translation and elongation of the amino acid sequence. However it has been hard to satisfactorily establish the structure of the bound ribosome stalk protein because of its flexibility. So, the researchers used the high resolution and fast image capture of high-speed atomic force microscopy to reveal its structure.
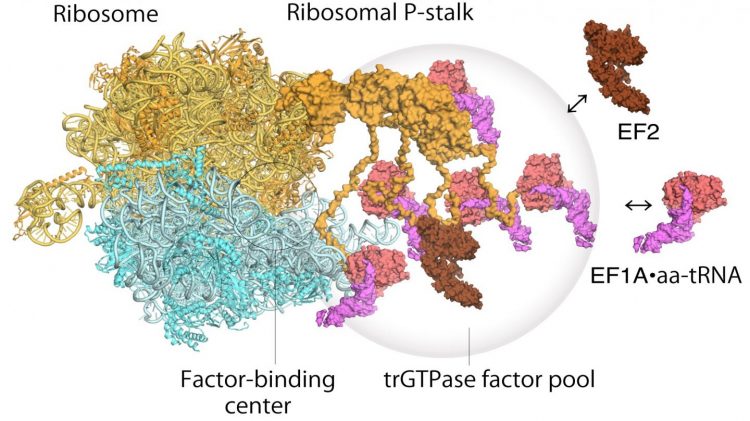
Model of translating ribosomes and elongation factors. EF1A•GTP•aatRNA and EF2 assemble to the ribosomal stalk on the translating ribosome. The translation factor pool contributes to efficient protein synthesis in a crowded intracellular environment [credit: Proceedings of the National Academy of Sciences].
Atomic force microscopy uses a nanoscale tip to feel samples. The team utilised the technique to show that the stalk protein actually flips between two conformations – one that agrees with previous structural models and one entirely unexpected new conformation.
As for how the ribosome operates, a two step mechanism had been previously proposed to describe how genetic information is translated through proteins known as “translational GTPase factors”. The first step is the recruitment of the factors to the factor-tethering site on the protein stalk, thereby increasing the concentration of factors there – so-called factor pooling. The second step is the binding and stabilising of a translational GTPase on the ribosomal factor-binding centre to catalyse GTPase hydrolysis. From their high speed atomic force microscopy study, the researchers were able to obtain the first visual evidence for the translational GTPase factor pooling mechanism by the ribosomal stalk.
Although the study was unable to give conclusive evidence of the action of the factors once bound, the researchers did note that the factors appeared to be retained in the vicinity once GTPase hydrolysis was complete, suggesting a potential role of the stalk protein in further stages of protein synthesis. The researchers conclude that “future work with HS-AFM will provide further important information to understand the dynamic behaviours of these complex translational machineries.”
The study was published in PNAS.
Related topics
Imaging, Label-Free, Microscopy
Related organisations
Kanazawa University, Niigata University