New immunosensor developed for use in immunoassays
Posted: 31 May 2021 | Victoria Rees (Drug Target Review) | No comments yet
A modified luciferase enzyme has been developed as an immunosensor to be fused with a Q-body and used in immunoassays.
Researchers from Tokyo Tech, Japan, have developed a novel immunosensor for use in immunoassays. The scientists used a modified luciferase enzyme called NanoLuc (Nluc) that is originally responsible for bioluminescence in shrimp. This immunosensor works on the bioluminescence resonance energy transfer (BRET) principle, so is termed BRET Q-body.
According to the researchers, immunosensors are widely used in immunoassays to detect antigens. One such immunosensor is a quenchbody (Q-body), which contains a modified antibody fragment with a quenched fluorescent dye. When an antigen binds to the Q-body, the dye leaves the antibody and the fluorescence intensifies. The change in fluorescence intensity is easy to measure, making Q-body-based antigen detection systems incredibly simple. However, this method requires an external light source to excite the electrons in the fluorescent dye to produce luminescence.
One way to solve this is to induce luminescence by an alternative method. To achieve this, the researchers used Nluc and fused it to the Q-body. Then, a luminescent substrate is added to the fused Q-body. The substrate reacts with the enzyme and this reaction provides the energy required by the dye to induce fluorescence.
“The BRET Q-body system can be used to visualise the presence or the absence of an antigen as a change in the emission colour without any instrument,” said Professor Hiroshi Ueda, who led the team of researchers.
To prepare the BRET Q-body, the researchers used a single-chain antibody fragment which binds to the antigen BGP, a protein found in the bone. The antibody fragment was then labelled with a fluorescent dye. They then tested the fluorescence intensity of the resulting BRET Q-bodies and observed that the addition of the antigen increased the intensity of the fluorescence.
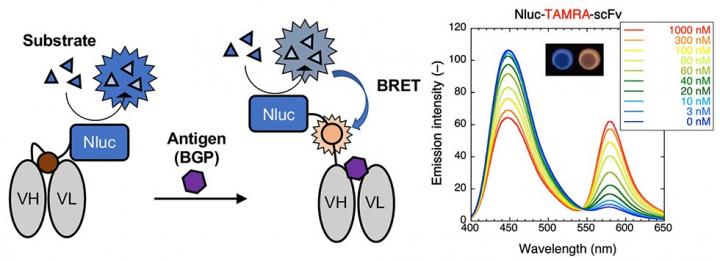
The enzyme “Nluc” is added to the Q-body forming a Bret Q-body. Luminescence is observed upon antigen binding. To the right, emission spectra in the presence of the substrate of the BRET-Q body labeled with the fluorescent dye TAMRA-C5-mal can be observed. The inset shows the change in color observed on antigen binding [credit: Tokyo Tech].
After these initial results, the researchers tested the antigen dependency of the BRET Q-body. They compared the fluorescence obtained by the new BRET-based method against the conventional irradiation-based method. The fluorescence intensity of the BRET-Q body was initially measured with excitation light and later in the presence of the luminescent substrate. They found that the antigen-binding brought the Nluc enzyme and the dye closer together resulting in higher fluorescence intensity levels when the substrate was used. As luminescence from the BRET Q-body is obtained initially from the enzyme and then from the fluorescent dye upon antigen binding, a simple colour change indicates the presence of an antigen.
The team say this study paves the way forward for a new class of bioluminescent sensors that do not require an external excitation light source in immunoassays.
Their findings were published in Analytical Chemistry.
Related organisations
Tokyo Tech
Related people
Professor Hiroshi Ueda