Enzyme PapB could boost diabetes and obesity peptide drugs
Posted: 20 October 2025 | Drug Target Review | No comments yet
Researchers have discovered an enzyme, PapB, that can ‘tie off’ therapeutic peptides into stable rings, which could help improve GLP-1 drugs for diabetes and obesity – making them more effective and longer lasting.

Researchers at the University of Utah have identified a new enzyme, called PapB, capable of ‘tying off’ therapeutic peptides into tight rings, a process known as macrocyclisation. This enzymatic method has the potential to create stronger, longer-lasting versions of GLP-1 medications such as semaglutide – the active ingredient in Ozempic and Wegovy – used to treat diabetes and obesity.
New enzyme enables next-gen peptide drugs
Creating cyclic peptides is highly valuable because the ring structures make drugs more stable, extend their duration in the body and improve their effectiveness on biological targets.
Peptides themselves can be extremely difficult to work with because they have a lot of reactive chemical handles
“Peptides themselves can be extremely difficult to work with because they have a lot of reactive chemical handles. But this is what makes them so great in biology. You can get the type of reaction that you want in the body, but it’s difficult to modify them in hyper-specific ways,” said Karsten Eastman, a research associate in the University’s Department of Chemistry and CEO and co-founder of Sethera Therapeutics. “What we show in the study is an enzymatic method – using a tiny molecular machine to modify or hyper modify peptides in extremely controlled ways – enabling what we believe will be next generation peptide therapeutics.”
From lab discovery to commercial venture
The study demonstrates that unlike traditional chemical methods for closing peptide rings, which can be expensive and tricky late in drug development, the newly discovered enzyme offers a much simpler alternative. PapB naturally forms a precise chemical bond that closes the peptide chain into a ring without the ‘leader’ sequences most enzymes require to recognise their targets.
The team used PapB to connect the ends of GLP-1-like peptides through a sulfur-carbon bond, known as a thioether. Laboratory tests confirmed the formation of these rings, even when the peptides contained nonstandard building blocks found in many modern incretin drugs.
“We were surprised by how flexible the enzyme turned out to be,” said Jake Pedigo, lead author and graduate student in the Bandarian lab. “It didn’t need the usual leader sequence and it still worked even when we swapped in unusual amino acids. That combination of precision and adaptability makes PapB a practical tool for peptide engineering.”
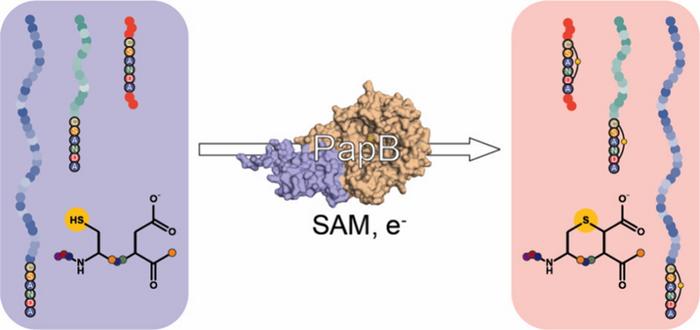

Graphic illustrates the PapB enzyme that ties off peptides. Credit: American Chemical Society and University of Utah
Enhancing therapeutic potential
The enzyme could improve peptide stability and therapeutic effectiveness by protecting peptides from proteases – enzymes that naturally digest proteins in the body.
You have these peptides that could have a great biological response, but if that biological response only lasts minutes, then all of a sudden you don’t have a good therapeutic
“You have these peptides that could have a great biological response, but if that biological response only lasts minutes, then all of a sudden you don’t have a good therapeutic,” Eastman said. “By using this enzymatic method to tie off the ends, we are essentially hiding the peptide from some of the most common proteases in the body –which are what breaks down peptides. This would enable the longer half-life.”
By demonstrating that PapB does not require a leader sequence, the researchers have shown it can be applied to a wide range of peptides, potentially creating hardier, more targeted therapeutics.
“Big pharma’s GLP-1 backbones are already excellent,” Eastman said. “What we’re adding is a clean, late-stage enzymatic step that can make those molecules work even harder. By installing a small, well-defined ring, we can tune how long the drug lasts, how stable it is and even how it signals – all while staying compatible with the complex structures already in use.”
Related topics
Analysis, Assays, Biotherapeutics, Drug Discovery, Drug Discovery Processes, Enzymes, Macrocyclic peptides, Obesity, Peptide Therapeutics, Screening, Stabilizing ring, Translational Science
Related organisations
The University of Utah