Airway-on-a-chip technology enables COVID-19 drug repurposing
Posted: 7 May 2021 | Victoria Rees (Drug Target Review) | No comments yet
An airway-on-a-chip has been used to show that amodiaquine inhibits SARS-CoV-2 infection, making it a potential COVID-19 therapeutic.
Researchers have used organ-on-a-chip (organ chip) technology to identify the antimalarial drug amodiaquine as a potent inhibitor of infection with SARS-CoV-2, the virus that causes COVID-19, as outlined in their paper published in Nature Biomedical Engineering.
According to the researchers, the organ chip-based drug testing ecosystem streamlines the process of evaluating the safety and efficacy of existing drugs for new medical applications, providing a proof-of-concept for the use of organ chips to rapidly repurpose existing drugs for new medical applications, including future pandemics.
The team from the Wyss Institute at Harvard, US, which developed the organ chip, examined eight existing drugs, including hydroxychloroquine and chloroquine, that have showed antiviral activity against SARS-CoV-2 in conventional cell culture assays. However, the researchers highlight that cells grown in a dish do not behave like the cells in a living human body, meaning that many drugs that appear effective in lab studies do not work in patients – hence the need for the organ chip.
Development of the chip technology
Over three years ago, the Wyss Institute was awarded funding from the Defense Advanced Research Projects Agency (DARPA) and US National Institutes of Health (NIH) to explore whether its human organ chip microfluidic culture technology, which mimics the function of human organs in vitro, could be used to confront potential biothreat challenges including pandemic respiratory viruses.
…amodiaquine prevented the transmission of the virus from sick to healthy animals more than 90 percent of the time”
The human airway chip that the team developed is a microfluidic device roughly the size of a USB memory stick that contains two parallel channels separated by a porous membrane. Human lung airway cells are grown in one channel that is perfused with air, while human blood vessel cells are grown in the other channel, which is perfused with liquid culture medium to replicate blood flow. Cells grown in this device naturally differentiate into multiple airway-specific cell types in proportions that are similar to those in the human airway and develop traits observed in living lungs such as cilia and the ability to produce and move mucus.
Two years into the project, the team was advancing its lung airway chip to study drugs that could be repurposed to treat influenza virus infections. Then, in January 2020, the direction for the group shifted when the news of the spread of SARS-CoV-2 became widespread.
“We closely followed the updates because we thought that our airway chip model could provide an important tool for studying this virus,” said Dr Longlong Si, one of the first authors on the study.
Use against SARS-CoV-2
According to the team, the airway chip cells have higher levels of angiotensin-converting enzyme 2 (ACE2) receptor protein, which plays a central role in lung physiology and is how COVID-19 can infect human cells.
“Our biggest challenge in shifting our focus to SARS-CoV-2 was that we do not have lab facilities with the necessary infrastructure to safely study dangerous pathogens. To get around that problem, we designed a SARS-CoV-2 pseudovirus that expresses the SARS-CoV-2 Spike (S) protein, so that we could identify drugs that interfere with the S protein’s ability to bind to human lung cells’ ACE2 receptors,” said Dr Haiqing Bai, another of the first authors. “A secondary goal was to demonstrate that these types of studies could be carried out by other organ chip researchers who similarly have this technology, but lack access to lab facilities required to study highly infectious viruses.”
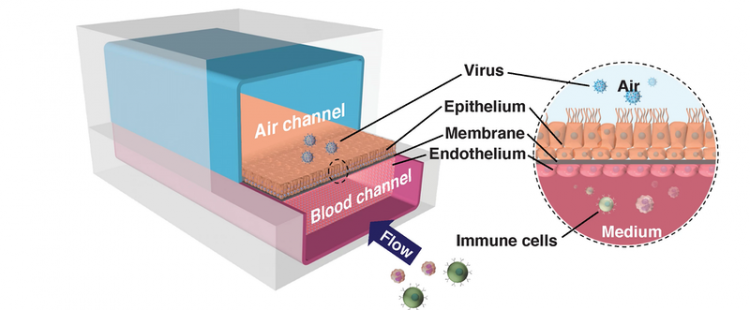
The human airway chip recreates the interface of the human lung’s airways and blood vessels, allowing researchers to study how different drugs and pathogens like viruses affect lung function [credit: Wyss Institute at Harvard University].
Using the pseudovirus, the team first perfused the chips’ blood vessel channel with several approved drugs, including amodiaquine, toremifene, clomiphene, chloroquine, hydroxychloroquine, arbidol, verapamil and amiodarone, all of which have exhibited antiviral activity against in previous studies. However, in contrast to static culture studies, the researchers were able to perfuse the drug through the channels of the chip using a clinically relevant dose to mimic how the drug would be distributed to tissues in the body. After 24 hours, they introduced the SARS-CoV-2 pseudovirus into the airway chips’ air channel to mimic infection by airborne viruses, like that in a cough or sneeze.
Only three of these drugs significantly prevented viral entry without producing cell damage in the airway chips: amodiaquine, toremifene and clomiphene. The most potent drug, amodiaquine, reduced infection by about 60 percent.
The team also performed spectrometry measurements to assess how the drugs impacted the airway cells. These studies revealed that amodiaquine produced distinct and broader protein changes than the other antimalarial drugs.
Lead optimisation
Despite the promise of amodiaquine, the team still needed to demonstrate that it worked against the real infectious SARS-CoV-2 virus. The researchers teamed up with Dr Matthew Frieman at the University of Maryland School of Medicine and Dr Benjamin tenOever at the Icahn School of Medicine at Mount Sinai, both US.
The Frieman lab tested amodiaquine and its active metabolite, desethylamodiaquine, against native SARS-CoV-2 via high-throughput assays in cells in vitro and confirmed that the drug inhibited viral infection.
In parallel, the tenOever lab tested amodiaquine and hydroxychloroquine against native SARS-CoV-2 in a comparison in a small animal COVID-19 model and saw that prophylactic treatment with amodiaquine resulted in approximately 70 percent reduction in viral load upon exposure, while hydroxychloroquine was ineffective. They also saw that amodiaquine prevented the transmission of the virus from sick to healthy animals more than 90 percent of the time and that it was also effective in reducing viral load when administered after introduction of the virus.
“Seeing how beautifully amodiaquine inhibited infection in the airway chip was extremely exciting,” said Frieman. “And, the fact that it seems to work both before and after exposure to SARS-CoV-2 means that it could potentially be effective in a wide variety of settings.”
Next steps
Since the identification of amodiaquine as a potential candidate to fight COVID-19, it has been included in a clinical trial in collaboration with the University of Witwatersrand, South Africa, and Shin Poong Pharmaceutical in South Korea. The Drugs for Neglected Diseases Initiative (DNDi) also added amodiaquine to the ANTICOV clinical trial for COVID-19, which spans 19 sites in over 13 different countries in Africa.
 In addition to SARS-CoV-2, the team are now also focusing on using the airway chip to develop drugs against other viral pathogens.
In addition to SARS-CoV-2, the team are now also focusing on using the airway chip to develop drugs against other viral pathogens.
“Thanks to our experience using this drug development pipeline to validate amodiaquine for COVID-19, we are now applying what we learned to influenza and other pandemic-causing pathogens,” said co-author Dr Ken Carlson. “This process has given us confidence that organ chips are predictive of what we see in more complex living models of viral infections.”
In addition to influenza, the team is now exploring drugs that could be used against the new SARS-CoV-2 mutant strains, to suppress the dangerous cytokine storm that leads to many hospitalisations and to relieve the symptoms of so-called long COVID.
Related topics
Drug Development, Drug Leads, Drug Repurposing, In Vitro, Organ-on-a-Chip
Related organisations
Defense Advanced Research Projects Agency (DARPA), Drugs for Neglected Diseases initiative (DNDi), Harvard's Wyss Institute, Icahn School of Medicine at Mount Sinai., Maryland University, Shin Poong Pharmaceutical, University of Witwatersrand, US National Institutes of Health (NIH)
Related people
Dr Benjamin tenOever, Dr Haiqing Bai, Dr Ken Carlson, Dr Longlong Si, Dr Matthew Frieman







