Silo Pharma initiates pre-IND package for ketamine to treat fibromyalgia
Posted: 13 October 2022 | Victoria Rees (Drug Target Review) | No comments yet
Silo Pharma says it is preparing a pre-IND package and meeting with the FDA for a topical formulation of ketamine to treat fibromyalgia.
Silo Pharma has announced that it is working on the preparation of a pre-Investigational New Drug (IND) package and meeting request with the US Food and Drug Administration (FDA) for a novel topical formulation of ketamine, designated as SPC-26, for the treatment of fibromyalgia.
According to the company, the submission of a pre-IND meeting request for collaborative discussions with the FDA will be made in anticipation of the filing a clinical IND package.
“We are confident that our highly constructive pre-clinical work on SP-26 will offer strong support for our pre-IND package as we seek to advance our time-released ketamine delivery system into the clinic,” said Eric Weisblum, Chief Executive Officer of Silo Pharma. “We intend to pursue the 505(b)(2) regulatory pathway.”
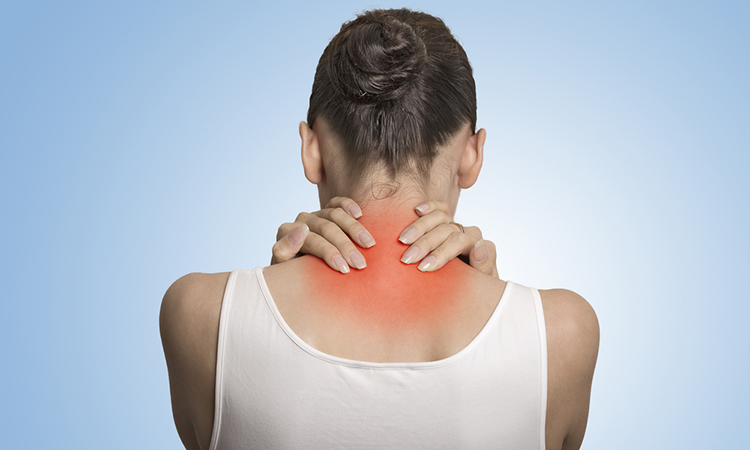
In collaboration with joint venture partner Zylö Therapeutics, Silo Pharma is pursuing the clinical development of ketamine using Zylö’s Z-pod™ technology. Pre-clinical studies have shown that the Z-pod can hold and distribute ketamine in a time-released manner and the company has determined to pursue fibromyalgia as an initial indication. Fibromyalgia is a chronic condition causing pain to the connective tissues through the body including muscles, ligaments and tendons. Musculoskeletal pain is often accompanied by sleep difficulties, fatigue, mood disorders and problems with memory and concentration.
NEWS: Clarametyx says the FDA has accepted an IND application for first-in-human trials of CMTX-101, an antibody to treat bacterial pneumonia…
Related topics
Clinical Trials, Drug Development, Legal & Compliance, Therapeutics, Translational Science
Related conditions
Fibromyalgia
Related organisations
Silo Pharma, US Food and Drug Administration (FDA), Zylö Therapeutics
Related people
Eric Weisblum