Researchers develop organoid models to study non-alcoholic fatty liver disease
Posted: 27 February 2023 | Ria Kakkad (Drug Target Review) | No comments yet
The team used these models to show drug responses and established a CRISPR-screening platform to identify potential therapeutic targets for non-alcoholic fatty liver disease.


Researchers from the Organoid Group (former Clevers group, Hubrecht Institute), together with scientists from the Princess Máxima Center, all Netherlands, established novel human organoid models of non-alcoholic fatty liver disease (NAFLD). They used these models to shed light on drug responses and established a CRISPR-screening platform to identify novel disease mediators and potential therapeutic targets. These models will aid in testing and developing novel medicines to treat NAFLD and help to get a better understanding of the disease biology. The results of the study was published in Nature Biotechnology.
No treatment for fatty liver currently exists that can halt or revert the disease. As the disease progresses, the risk of irreversible liver damage and the need for liver transplantation greatly increases. In addition, individuals with fatty liver are at heightened risk to develop liver cancer. Identifying ways to tackle the disease is very challenging due to the scarcity of model systems. Mice differ greatly in their metabolism and can therefore not be used as a representative model system of the human disease. Moreover, current human-based in vitro models possess several drawbacks. Genetic modification of these models is difficult, and it is currently impossible to quickly generate large numbers of cells.
Now, the researchers turned to organoids to establish three models that capture different triggers of fatty liver development. Firstly, they “fed” the organoids with a mixture of fatty acids to mimic a Western diet and witnessed the rapid development of fatty liver organoids. As a second model, the team introduced the top risk mutation for fatty liver disease into their organoid system using a new CRISPR tool named prime editing. Organoids with this mutation displayed much more severe fat accumulation than organoids without it. Finally, the researchers also modelled genetic lipid disorders using CRISPR-Cas9 to investigate how these disorders influence the development of fatty liver disease. These mutant organoids spontaneously developed severe fatty livers because of a build-up of sugar-derived fats.
The team then screened many drug candidates to treat NAFLD on the newly developed organoid models. Interestingly, the researchers observed that the different fatty liver organoid models responded to the drugs in a very comparable manner. Doing so, they identified a subset of drugs that were effective across all models. Interestingly, these effective drugs functioned by a common mechanism in which the generation of lipids from sugars was blocked. Importantly, the team also observed that organoids having the top risk mutation for fatty liver disease did not react to all drugs in the same way as organoids without the mutation. This shows the organoids can be used as a tool for personalised medicine.
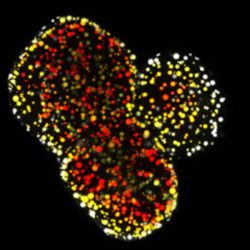

Modelling a genetic lipid disorder leads to the development of spontaneous fatty liver organoids. Lipids are depth-coloured
[Delilah Hendriks & Benedetta Artegiani. Copyright Hubrecht Institute].
The researchers went on to use their organoid models to establish a genetic screening platform to identify novel genes with roles in NAFLD. The researchers turned their organoids into a CRISPR-screening platform, named FatTracer. They used this platform to investigate the effect of loss of specific genes on the fatty liver phenotype, which could be visualised in real-time over 20 days. After screening of 35 candidates, a novel and critical role for the FADS2 gene (fatty acid desaturase 2) in NAFLD was discovered. Disruption of FADS2 made the organoids much more fatty. The team wondered whether the opposite condition, having more FADS2, would instead be beneficial to the disease. Indeed, when overexpressing FADS2, the fatty liver that the organoids once displayed was severely reduced, suggesting it is a potential novel therapeutic target.
These novel fatty liver organoid models pave the way for many future directions. For example, the researchers would like to better understand the genetic risks that are linked to the development of fatty liver, as well as to study what factors influence disease progression. The aim is to use these models to define personalised drug therapies that can cure the liver from fat overload.
Related topics
CRISPR, Disease Research, Drug Discovery, Gene Testing, Genomics, Organoids, Personalised Medicine, Screening
Related conditions
non-alcoholic fatty liver disease (NAFLD)
Related organisations
Hubrecht Institute, Princess Máxima Center