Vital cell metabolic pathway regulator identified by study
Posted: 19 September 2019 | Victoria Rees (Drug Target Review) | No comments yet
A study has revealed that the mitoNEET protein controls a metabolic and functional gateway on mitochondria, which could provide a drug target for cancer.

A research team has discovered how a cancer-linked protein can manipulate gateways on the surface of mitochondria. They say that developing a drug to control this could be effective as a cancer treatment.
…controlling the regulator of metabolic and functional interactions of VDAC with a drug could be useful against several kinds of cancer”
The study was conducted by Rice University, the University of California, San Diego, the University of Missouri-Columbia, all US and the Hebrew University of Jerusalem, Israel.
The mitoNEET protein naturally adheres to the outer surface of the mitochondria and helps to regulate cells by controlling reduction-oxidation, or redox processes, and metabolism of cells. It has been shown to play a role in cancer, but how was unknown.
The researchers revealed how mitoNEET can close the primary gateway on the surface of mitochondria, which supply cells with chemical energy. These voltage-dependent anion channels (VDACs) open and close to allow the passage of metabolites and other small molecules.
“The VDAC channel transports all types of metabolites between the cytosol and the mitochondria,” said study co-author José Onuchic, a physicist and co-director of Rice University’s Center for Theoretical Biological Physics (CTBP). “Dysfunction of this channel is involved in many diseases including cancer and fatty liver disease.”
The researchers suggest that controlling the regulator of metabolic and functional interactions of VDAC with a drug could be useful against several kinds of cancer.
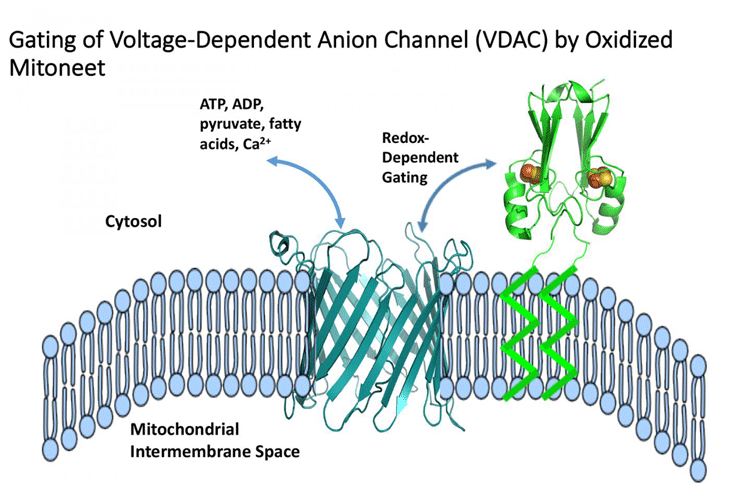

When oxidised, the protein mitoNEET (green) can close voltage-dependent anion channels, or VDACs (centre), passageways that allow metabolites and signalling molecules to pass through the outer membrane (blue band) of the mitochondria, the ‘power plant’ that supplies cells with chemical energy (credit: CTBP/Rice University).
Co-author Patricia Jennings, a structural biologist at UCSD, said, “The discovery that mitoNEET directly gates VDAC, the major porin of mitochondria, as well as the accompanying structural analysis and predictions for this interaction, affords a new platform for investigations of methods to induce cancer cells to commit cell suicide, or apoptosis/ferroptosis, in a cancer-specific, regulated process.”
The findings were published in the Proceedings of the National Academy of Sciences.
Related topics
Disease Research, Drug Targets, Metabolomics, Oncology, Protein, Research & Development
Related conditions
Cancer
Related organisations
Hebrew University of Jerusalem, Rice University, University of California San Diego, University of Missouri-Columbia
Related people
José Onuchic, Patricia Jennings