Potential target to control T follicular helper cells identified
Posted: 23 June 2020 | Victoria Rees (Drug Target Review) | No comments yet
A new study shows how the Bcl6 protein can regulate T follicular helper cells, presenting a target for autoimmune and infectious diseases.

Scientists at the La Jolla Institute for Immunology (LJI), US, have discovered a potential new way to fight a range of infectious diseases, cancers and autoimmune diseases. The study reveals how the Bcl6 protein works as a ‘master regulator’ in the immune system’s T follicular helper (Tfh) cells, so is a target.
The researchers say that their findings are an important step toward designing vaccines and therapies that can ‘switch on’ the immune cells that help produce disease-fighting antibodies. Scientists may also be able to ‘switch off’ these cells to counteract immune cells dysfunction in autoimmune diseases, the team say.
“This cell type (Tfh cells) sometimes does bad things in autoimmune diseases – particularly autoantibody diseases like lupus, rheumatoid arthritis and Sjogren’s syndrome,” said LJI investigator Dr Shane Crotty, who led the research. “So, hopefully, our fundamental knowledge about the circuitry of this cell can help us understand how to turn it off in autoimmune diseases.”
In 2009, Crotty’s laboratory published work showing that a protein called Bcl6 controls how helper T cells differentiate to do different jobs in the body. They found that Bcl6 prompts helper T cells to become Tfh cells, which work with B cells to produce powerful antibodies.
“There is great interest in the use of Tfh-cell-associated biology for enhancement of vaccines,” said Crotty. “There is also great interest in targeting Tfh cell-associated biology for therapeutic interventions in human autoimmune diseases, allergies, atherosclerosis, organ transplants and cancer.”
For the new study, Crotty led a complex effort to test competing theories for how Bcl6 controls Tfh. The researchers used mouse models and a range of genetic sequencing tools to determine that Tfh cells actually need Bcl6 to even exist.
They found that Bcl6 acts mainly as a repressor in helper T cells, meaning that it blocks the expression of other proteins in these cells through a series of genetic switches, which they mapped. These showed that Bcl6 controls a ‘double negative circuit’.
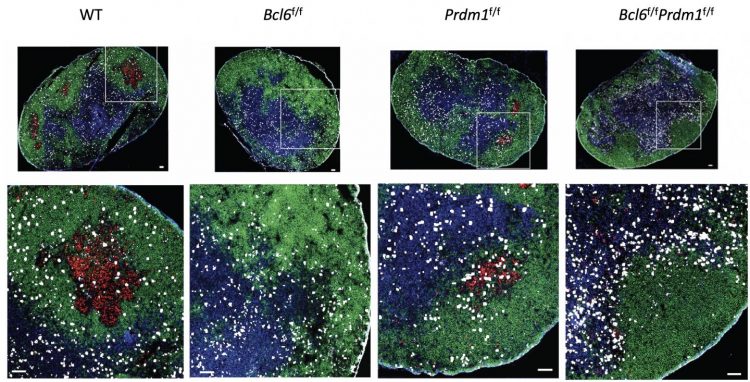

Bcl6 prompts helper T cells to become T follicular helper cells (Tfh cells, shown in blue), which work with B cells (shown in green) to produce powerful antibodies. Activated B cells give rise germinal centers (shown in red), where mature B cells proliferate and produce highly specific antibodies against pathogens. Bcl6 blocks the expression of two proteins that normally stop Tfh cell differentiation. When Bcl6 does its job, helper T cells are free to become Tfh cells when the body needs them [credit: Jinyong Choi, La Jolla Institute for Immunology].
“The protein Bcl6 switches this cell type on, but it is a protein that is only known to switch things off. So, we did a lot of experiments to figure out that it controls cells by a series of double negatives. It turns off genes that turn off other genes,” explained Crotty.
Bcl6 blocks the expression of two proteins that normally stop Tfh cell differentiation. When Bcl6 does its job, helper T cells are free to become Tfh cells when the body needs them.
“Increasing emphasis will surely now be placed on how to apply that knowledge to Tfh-related therapeutics,” Crotty said, adding that tweaking immune responses through Bcl6 could help control autoimmune diseases such as multiple sclerosis and type 1 diabetes.
The study was published in Nature Immunology.
Related topics
Drug Targets, Immunology, Immunotherapy, Research & Development, T cells, Targets
Related conditions
Cancer, Lupus, rheumatoid arthritis, Sjögren's Syndrome
Related organisations
La Jolla Institute for Immunology (LJI)
Related people
Dr Shane Crotty