New virus-like probes could make COVID-19 tests more accurate and reliable
Posted: 2 December 2020 | Hannah Balfour (Drug Target Review) | No comments yet
The novel probes, known as positive controls, could make it easier to validate rapid, point-of-care diagnostic tests for COVID-19 across the globe.
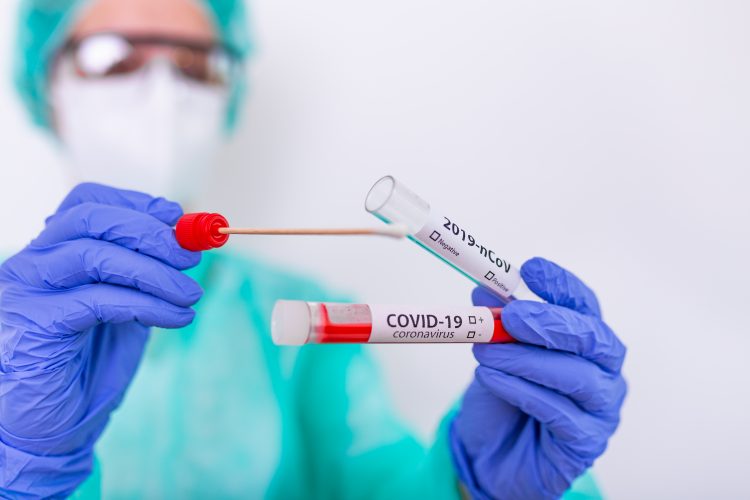

Researchers have developed two novel virus-like probes, called positive controls, for validating COVID-19 tests. The team suggest their controls more stable, simpler to manufacture and cheaper than the controls currently used for COVID-19 tests.
Positive controls are used to validate tests and experiments, making them an essential part of the laboratory toolkit. However, scientists have suggested that there may be an issue with the controls currently used to validate COVID-19 tests. According to nanoengineers from the University of California – San Diego (UC San Diego), most probes used to validate rapid COVID-19 tests are naked synthetic RNAs, plasmids or RNA samples from infected patients, which can degrade easily and require refrigeration to maintain activity. The scientists said this makes them inconvenient and costly to ship globally and/or store for prolonged periods.
In a new study published in ACS Nano, researchers from the UC San Diego Jacobs School of Engineering sought to design positive controls that are more stable, simpler to manufacture and cheaper. The paper details the development and testing of two different controls made from virus-like particles expressing segments of RNA from the SARS-CoV-2 virus (which causes COVID-19).
“Our goal is to make an impact not necessarily in the hospital, where you have state-of-the-art facilities, but in low-resource, underserved areas that may not have the sophisticated infrastructure or trained personnel,” said Nicole Steinmetz, leader of the study and a professor of nanoengineering at the UC San Diego Jacobs School of Engineering.
The team developed two different controls: one made from plant virus nanoparticles, the other from bacteriophage nanoparticles. Both of which, according to the scientists, can be stored for a week at temperatures up to 40°C and retain 70 percent of their activity even after one month of storage. They can also pass detection as the SARS-CoV-2 coronavirus without being infectious.
The plant virus-based controls use the cowpea chlorotic mottle virus, which infects black-eyed pea plants. To develop the probe, the researchers opened the virus, removed its RNA contents and replaced them with a synthesised RNA template containing specific sequences from the SARS-CoV-2 virus, before closing the shell of the virus.
To make the bacteriophage-based controls, the researchers inserted gene sequences of interest from the SARS-CoV-2 virus and genes coding for surface proteins of the bacteriophage Qbeta into plasmids. When these plasmids are introduced into bacteria, they reprogramme them to produce virus-like particles with SARS-CoV-2 RNA sequences on the inside and Qbeta bacteriophage proteins on the outside.
Both controls were validated with clinical samples.
According to the researchers, one advantage of their probes is that, unlike the COVID-19 test controls currently in use, they can be used in all steps of a COVID-19 test in parallel with patient samples. The team says this would make it easier to identify if something went wrong during the initial stages of analysis.
So far, the researchers have adapted their controls for use in the US Centre for Disease Control and Prevention (CDC)-authorised RT-PCR test. While this is currently the gold standard for COVID-19 testing, the team say it is expensive, complex and can take days to return results due to the need for specialist lab equipment.
The nanoengineers are now adapting their controls for use in less complex COVID-19 diagnostic tests, such as the RT-LAMP test that can be done on the spot, out of the lab and provide immediate results.
Steinmetz concluded: “It is a relatively simple nanotechnology approach to make low-tech assays more accurate. This could help break down some of the barriers to mass testing of underserved populations in the US and across the world.”
Related topics
Analytical Techniques, Bioengineering, Immunology, Nanoparticles, Nanotechnology, Protein, Protein Expression, Proteomics, Research & Development, Screening, Target Validation, Technology
Related conditions
Coronavirus, Covid-19
Related organisations
University of California - San Diego (UC San Diego)
Related people
Nicole Steinmetz