Is an HIV vaccine on the horizon?
Posted: 10 October 2022 | Victoria Rees (Drug Target Review) | No comments yet
Researchers in the US have developed a potential HIV vaccine approach that aims to prompt the creation of broadly neutralising antibodies via mRNA.


HIV is a virus that attacks the body’s immune system. If not treated, it can lead to AIDS and there is no effective cure at present. HIV therefore remains a key focus on many researchers in the drug discovery industry.
Now, scientists from Scripps Research, the International AIDS Vaccine Initiative (IAVI), the Ragon Institute and Moderna have made critical advances in developing an effective vaccine against HIV.
The findings, published in Immunity in two individual papers, describe the first steps in a vaccine approach that aims to prompt the creation of broadly neutralising antibodies (bnAbs) — antibodies that are broad enough to fight and protect against many different variants of a virus. By identifying the most promising bnAbs and the human genes needed to make them — as well as designing protein and messenger RNA (mRNA) vaccine candidates to begin bnAb creation and verifying the vaccine candidates — the team is paving the way to create an effective HIV vaccine.
“Our two studies describe a collaborative effort to genetically and structurally understand bnAbs and ultimately ‘reverse engineer’ vaccines to elicit these bnAbs,” said senior author Dr William Schief, a Scripps Research professor and executive director of vaccine design at IAVI’s Neutralizing Antibody Center at Scripps Research. “HIV has remained one of the most difficult viruses to protect against because of its natural ability to quickly mutate and evade capture from the immune system. Working closely together across scientific disciplines and institutions, our team’s findings mark a crucial step forward in overcoming these historic hurdles and creating an effective HIV vaccine.”
Designing the vaccine
Researchers have studied how a small percentage of infected individuals with HIV are able to make bnAbs. Even when bnAbs do develop during infection in these cases, they arise too late to help block the virus. However, scientists have demonstrated that bnAbs can protect against the virus if they are present before a person gets infected with HIV. This observation has led researchers to try and develop vaccines that induce bnAbs in healthy individuals, but designing such vaccines has proved difficult.
…the researchers are continuing refine their vaccine approach and test it in additional models”
The new work aims to overcome this challenge by carefully choosing the bnAbs to elicit and then designing custom vaccines that coax the immune system to produce the target bnAbs in a stepwise manner. The team focused on bnAbs that bind to the apex of the HIV spike protein (analogous to the spike protein of SARS-CoV-2). These apex bnAbs employ extremely long loops called HCDR3 loops to pierce the spike protein like a spear. By binding to the apex of the HIV spike, the bnAbs prevent HIV from infecting human cells.
Testing the theory
The scientists needed to determine if the general population had the precursor cells that would eventually lead to the creation of these potent bnAbs.
“Finding the bnAbs we need is like searching for a needle in a haystack,” said co-first author of both papers Dr Zachary Berndsen, assistant professor of biochemistry at the University of Missouri. “To make an effective vaccine, we must first find the precursor antibodies that can eventually become bnAbs, while also seeing if those precursor antibodies are common enough throughout the general population to stimulate.”
All bnAbs mature from a type of antibody-producing white blood cell, called a naïve B cell. These B cells are often referred to as “germline” precursors. Through bioinformatic analyses of a massive database of 1.2 billion human antibody sequences, the researchers learned that two apex bnAbs had the most frequent precursor B cells. This discovery led the team to focus on developing a vaccine tailored to induce those two types of bnAb.
“The long HCDR3 loops in apex bnAbs are rare in human antibodies, thus one might expect that precursors to these apex bnAbs are also rare,” said co-first author Dr Jordan Willis, senior principal scientist at the IAVI Neutralizing Antibody Center at Scripps Research. “However, we found that two classes of apex bnAbs had relatively common precursors, so we decided to focus our vaccine design strategy on those two bnAbs.”
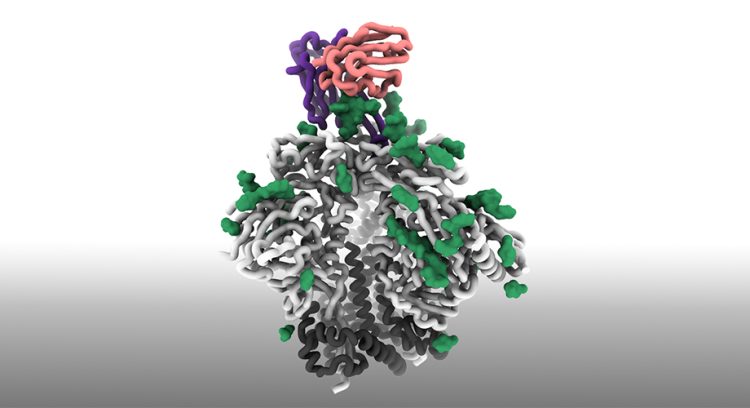

Scripps Research and collaborators demonstrate the first steps in an HIV vaccine approach that aims to prompt the creation of highly protective broadly neutralising antibodies. Pictured is the cryo-EM structure of the engineered priming immunogen (grey, black); a key feature of the HIV spike protein called glycans (green); and the precursor antibody (purple and pink) that it binds to with high affinity [credit: Scripps Research].
The researchers then found that natural HIV spike proteins do not bind to the germline precursors of the two target bnAbs, meaning that using a natural HIV spike protein as a vaccine would not induce the desired bnAb responses. To solve that problem, the researchers engineered a modified HIV spike protein that can bind to the germline precursors for both target bnAbs. This engineered spike protein serves as the “priming immunogen”, the first shot of the vaccine that binds and activates the right precursor B cells to start the process of producing the desired apex bnAbs.
“The first step in designing a vaccine to induce these apex bnAbs was to engineer a priming immunogen with a high binding affinity to the right germline precursors,” said co-first author Krystal Ma, a PhD graduate student at Scripps Research. “The next steps will be to design a series of boosting immunogens that can mature the precursors into bnAbs.”
After many rounds of design and extensive in vitro testing — combined with cryo-electron microscopy ‘snapshots’, which provided insight into how the virus may eventually fight back against the bnAbs — the team created a successful priming immunogen.
The final steps
The scientists demonstrates that the new immunogen was able to successfully bind to the germline precursor B cells and elicit the desired responses in mice expressing the bnAb germline genes at the same low frequency they appear in humans.
…the team is paving the way to create an effective HIV vaccine”
“This is a very important step, as it shows that vaccinating with our immunogen can actually elicit responses from the precursors we were targeting,” added Willis. “We also showed that vaccinating with an unmodified HIV protein could not elicit those responses, which proves that our affinity engineering was required.”
The antibody responses have potential to develop into bnAbs that could fight HIV, which the scientists will attempt to achieve in future studies with different boost immunogens that are still in the design stage.
As a successful vaccine needs to be viable from a manufacturing perspective as well, the team collaborated with scientists from Moderna to successfully formulate their germline-targeting immunogens into mRNA vaccines. This approach gave even better antibody responses in the mice than the typical vaccine formulation, while also being much easier and faster to manufacture.
With this validation in hand, the researchers are continuing refine their vaccine approach and test it in additional models with the intent to ultimately enter the clinic for human trials.
Related topics
Antibodies, Immunology, RNAs, Translational Science, Vaccine
Related organisations
International AIDS Vaccine Initiative (IAVI), Moderna, Ragon Institute, Scripps Research, University of Missouri
Related people
Dr Jordan Willis, Dr William Schief, Dr Zachary Berndsen, Krystal Ma