Organoid system simulates human liver-islet axis in diabetes
Posted: 26 January 2022 | Victoria Rees (Drug Target Review) | No comments yet
Scientists have developed a multi-organoid system from stem cells that can replicate the liver-islet axis in type 2 diabetes.

A group of researchers from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has developed a multi-organoid system from human induced pluripotent stem cells (hiPSCs) to simulate the human liver-islet axis in type 2 diabetes.
According to the researchers, type 2 diabetes is a systematic multi-organ metabolic disease, which is characterised by dynamic interplay among different organs. Pancreatic islet-liver axis is closely associated with normal glucose regulation and homeostasis maintenance. The dysfunction of the interplay between these organs may lead to type 2 diabetes progression.
“Stem cell-derived organoids, a new class of three-dimensional (3D) tissues, has the key structural and functional features of in vivo counterparts,” said Professor Qin Jianhua.
The team say that this newly developed multi-organoid system, outlined in a study published in Advanced Science, could simulate human liver-pancreatic islet axis in physiological and pathological conditions and enable 3D co-culture of hiPSC-derived liver and islet organoids for up to 30 days.
The researchers found that the generated liver and islet organoids exhibited favourable growth and improved tissue-specific functions in this perfused microfluidic 3D culture system.
“The sensitive glucose-stimulated insulin secretion was from the islet organoids and it increased glucose utilisation in the liver organoids,” said Qin, highlighting that this reflects the co-operative interaction between the two organs in this perfused co-culture system.
Transcriptional analyses revealed that the activated signalling pathways were associated with glucose/CYP450 metabolism and glycolysis/gluconeogenesis in liver and islet organoids.
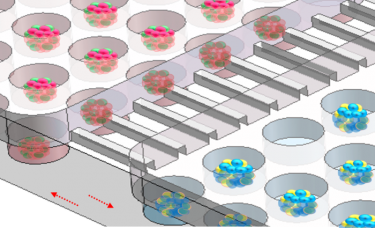
Enlarged view of the co-culture system [credit: Advanced Science].
Additionally, this microfluidic islet-liver organoid system exhibited mitochondrial dysfunction and decreased glucose transport capacity under hyperglycaemic conditions, which might be attributed to the alleviation by treatment with an antidiabetic drug.
“This novel system can not only reflect the feedback loop within the liver-islet axis, but also exhibit relevant responses to high glucose and antidiabetic drug, which is not easily achieved by conventional cell culture and animal models,” said Qin. “It will provide a unique platform for the study of complex type 2 diabetes pathogenesis and drug development.”
Related topics
Disease Research, Microfluidic Technology, Organoids, Stem Cells
Related conditions
Diabetes, Type-2 diabetes
Related organisations
Chinese Academy of Sciences (CAS)
Related people
Professor Qin Jianhua