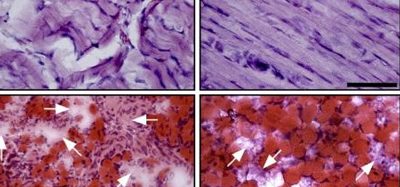
Mystery behind odd-shaped blood cancer cells revealed
Posted: 5 April 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Researchers have found that the Lamin B1 mutation causes odd-shaped nuclei in blood cancer cells, which may lead to improved care for leukaemia patients.
Scientists from the University School of Medicine in Seattle, US have discovered why the nuclei of certain blood cancer cells have a distinctly odd shape. The study, which was recently published in Cell Stem Cell, highlights clues on the origins and progression of these cancers and could suggest ways to diagnose and treat certain leukaemias at an earlier stage.
The distinct nuclei, called Pelger-Huët anomalies, were first observed under a microscope in 1928. Checking for this cellular aberration has long helped clinical labs diagnose leukaemia and myelodysplastic syndrome, a disease of blood-forming cells in the bone marrow. Although this structural change inside blood cells indicates possible cancer, until a recent study, it was unclear what caused it to happen.
“The primary diagnosis of many cancers, even in the era of genomic medicine, remains centred on the appearance of cells under a microscope.” said Dr Sergei Doulatov, an Associate Professor of medicine and senior investigator on the recent gene study, a collaborative work among several institutions. He also explained smear tests are an example of cancer screenings that look for irregularly shaped nuclei in a patient’s cells.
While they are still in their multipotent state and able to give rise to any one of a variety of blood cell categories, the progenitor cells for neutrophils, red blood cells or platelets are called myeloid cells. Myeloid cells themselves can sometimes show abnormal, pre-cancerous changes. The researchers on this recent study were able to cast suspicion on the loss of nuclear Lamin B1, encoded on chromosome 5q. It is frequently deleted in cells examined from abnormally growing myeloid tissue. Evidence from this study suggests this loss is at fault in the misshapen nuclei.
“Lamins are proteins that line the inside of the nucleus and are mutated in inherited disorders – famously progeria, the disorder of accelerated ageing,” explained Doulatov. Lamin protein production is also often dysregulated in cancers. “We showed that loss of nuclear Lamin B1 induces defects in the nuclear morphology and in human hematopoietic stem cells associated with malignancy,” the researchers noted.
His group went on to detail that Lamin B1 deficiency alters genome organisation. This in turn caused expansion of the blood-forming stem cells, a bias towards their becoming myeloids, genome instability due to defective DNA damage repair and other problems that set the stage for cancer. They also showed that the abnormal nuclei in the cells of myeloid pre-cancerous growths in patients were associated with deletions in chromosome 5q that spanned the lamin1 B1 region.
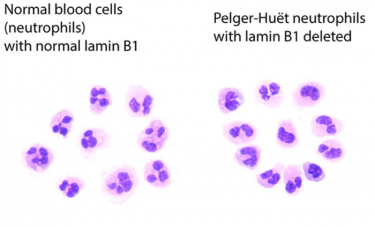
A comparison of normal neutrophils on the left, with blood cells with Pelger-Huët anomalies on the right
[Credit: Sergei Doulatov Lab].
Lamin B1 loss was both necessary and sufficient to cause the Pelger-Huët anomalies, according to the researchers. The scientists were also able to link this abnormal nuclear shape with progenitor and blood-forming stem cell fate determination through the organisation of the genome.
“We show that Lamin B1 deletion causes changes in stem cell function, nuclear shape and leukaemia progression. Our research discovers Lamin mutations in cancer and demonstrates that these mutations are responsible for the oddly shaped nuclei that have puzzled and helped pathologists recognise cancers over the past century,” concluded Doulatov.
The latest genomic findings and their consequences for aberrant transformations in blood-forming cells may be important in the future of leukaemia care. The presence of these changes, the researchers said, may be an early cancer biomarker, whose detection could permit for earlier diagnosis and treatment of leukaemia.
Related topics
Disease Research, Genome Editing, Genomics, Microscopy, Oncology, Stem Cells, Therapeutics
Related conditions
Leukaemia
Related organisations
University School of Medicine in Seattle
Related people
Dr Sergei Doulatov