Scientists create a nanobody that could treat Parkinson’s disease
Posted: 1 August 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Researchers have developed a nanobody that can penetrate the tough exterior of brain cells and untangle misshapen proteins that lead to Parkinson’s disease.


Researchers at Johns Hopkins Medicine, US have developed a nanobody capable of getting through the tough exterior of brain cells and untangling misshapen proteins that lead to Parkinson’s disease, Lewy body dementia, and other neurocognitive disorders caused by the damaging protein. The study was recently published in Nature Communications.
The team’s aim was to find a new type of treatment that could specifically target the misshapen proteins, called alpha-synuclein, which tend to clump together and clog the inner workings of brain cells. Emerging evidence has shown that the alpha-synuclein clumps can spread from the gut or nose to the brain, driving the disease progression.
In theory, antibodies have potential for zeroing in on clumping alpha-synuclein proteins, but the pathogen-fighting compounds have a hard time getting through the outer covering of brain cells. To squeeze through tough brain cell coatings, the researchers decided to use nanobodies, the smaller version of antibodies.
Traditionally, nanobodies generated outside of the cell may not perform the same function inside the cell. So, the researchers had to place nanobodies to help them keep stable within a brain cell. To do this, they genetically engineered the nanobodies to rid them of chemical bonds that typically degrade inside a cell. Tests showed that without the bonds the nanobody remained stable and was still able to bind to misshapen alpha-synuclein.
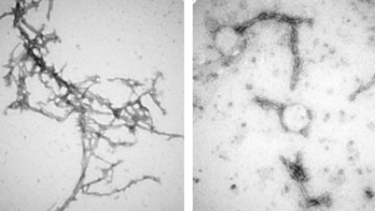

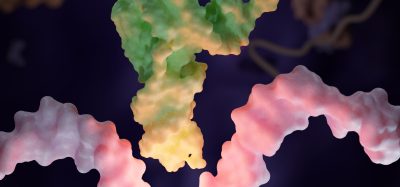
The structure of alpha-synuclein clumps (on the left) was disrupted by the nanobody PFFNB2 (as shown on the right)
[Credit: Xiaobo Mao],
The team made seven, similar types of nanobodies, known as PFFNBs, that could bind to alpha-synuclein clumps. Of the nanobodies they created, one — PFFNB2 — did the best job of glomming onto alpha-synuclein clumps but not single molecules, or monomer of alpha-synuclein. Monomer versions of alpha-synuclein are not harmful and may have important functions in brain cells. The researchers also needed to determine if the PFFNB2 nanobody could remain stable and work inside brain cells. The team found that in live mouse-brain cells and tissue, PFFNB2 was stable and showed a strong affinity to alpha-synuclein clumps rather than single alpha-synuclein monomers.
Additional tests in mice showed that the PFFNB2 nanobody cannot prevent alpha-synuclein from collecting into clumps, but it can disrupt and destabilise the structure of existing clumps.
“Strikingly, we induced PFFNB2 expression in the cortex, and it prevented alpha-synuclein clumps from spreading to the mouse brain’s cortex, the region responsible for cognition, movement, personality and other high-order processes,” said Dr Ramhari Kumbhar, co-first author of the study.
“The success of PFFNB2 in binding harmful alpha-synuclein clumps in increasingly complex environments indicates that the nanobody could be key to helping scientists study these diseases and eventually develop new treatments,” concluded Dr Xiaobo Mao.
Related topics
Antibodies, Disease Research, Nanoparticles, Nanotechnology, Neurosciences, Therapeutics
Related conditions
Lewy body dementia, Parkinson's disease
Related organisations
Johns Hopkins Medicine
Related people
Dr Ramhari Kumbhar, Dr Xiaobo Mao