Study reveals how tau impairs communication between neurons in Alzheimer’s
Posted: 21 September 2022 | Victoria Rees (Drug Target Review) | No comments yet
A new study has shown how tau weakens transmission across synapses in the brains of mice, providing insight into Alzheimer’s.


A team of scientists from the Okinawa Institute of Science and Technology (OIST), Japan, has revealed how excess tau – a key protein implicated in Alzheimer’s disease – can impair signalling between neurons in the brains of mice. According to the researchers, the study could open new pathways for treating the symptoms and potentially halting the progression of Alzheimer’s disease and other neurodegenerative disorders.
The team say that tau is produced in neurons, where it binds to and promotes the assembly of microtubules – long, thin filaments that maintain cell structure and provide pathways for transport within the cell. Tau usually exists in either this bound state or it is dissolved in the liquid that fills the cell. However, in some neurological disorders, particularly in Alzheimer’s disease, levels of soluble tau in certain brain regions become too high and it aggregates into insoluble structures called neurofibrillary tangles.
“A lot of scientists focus on the impact of these visible neurofibrillary tangles that are a hallmark of Alzheimer’s, but actually, it is the invisible levels of soluble tau that correlate most closely with cognitive decline,” said Professor Tomoyuki Takahashi, senior author of the study, published in eLife.
The research began ten years ago, when Takahashi’s team looked at the effect of high levels of soluble tau on signal transmission at the calyx of Held – the largest synapse in mammalian brains. When an electrical signal arrives at the end of a presynaptic neuron, neurotransmitters are released from membrane ‘packets’ called vesicles into the gap between neurons. When the neurotransmitters reach the postsynaptic neuron, they trigger a new electrical signal.
Using mice, the researchers injected soluble tau into the presynaptic terminal at the calyx of Held and found that electrical signals generated in the postsynaptic neuron dramatically decreased. They then fluorescently labelled tau and microtubules and saw that the injected tau caused new assembly of many microtubules in the presynaptic terminal. However, when they injected a mutant tau protein instead that lacked the binding site necessary to assemble microtubules, there was no effect on synaptic transmission.
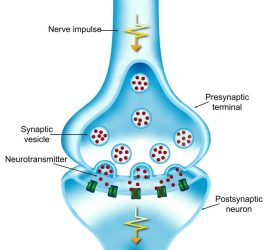

When an electrical signal arrives at the presynaptic terminal, neurotransmitters are released from vesicles into the gap between neurons. The neurotransmitters then trigger an electrical signal in the postsynaptic neuron [credit: OIST].
“This told us that the decrease in synaptic signalling was clearly linked to these newly assembled microtubules,” explained Takahashi.
The team also found that elevated tau decreased the transmission of high-frequency signals, while low-frequency transmission remained unchanged. High-frequency signals are typically involved in cognition and movement control. They therefore suspected that such a selective impact on high-frequency transmission might be due to a block on vesicle recycling, a vital process for the release of neurotransmitters across the synapse since synaptic vesicles must fuse with the presynaptic terminal membrane, in a process called exocytosis. These vesicles are then reformed by endocytosis and refilled with neurotransmitter to be reused. If any of the steps in vesicle recycling are blocked, it quickly weakens high-frequency signals, which require the exocytosis of many vesicles.
The scientists found that high levels of soluble tau primarily impaired endocytosis. The lack of reformed vesicles impaired recycling and eventually slowed down exocytosis as a secondary effect.
Importantly, the researchers found that a drug called nocodazole, which blocks new microtubule assembly, prevented injected tau from impairing endocytosis, impacting the development of Alzheimer’s.
While investigating a potential link between microtubules and endocytosis, the team realised that dynamin, a large protein that cuts off vesicles from the surface membrane at the final step of endocytosis, was discovered as a protein that binds to microtubules, although little is known about the binding site. When they fluorescently labelled tau, microtubules and dynamin, the researchers found that presynaptic terminals that had been injected with tau showed an increase of bound dynamin, preventing the protein from carrying out its role in endocytosis.
Finally, the team created many peptides with matching sequences of amino acids to parts of the dynamin protein, to see if any of them could prevent dynamin from binding to the microtubules and therefore rescue the signalling defects caused by tau protein. When one of these peptides, called PHDP5, was injected along with tau, endocytosis and synaptic transmission remained close to a normal level.
Moving forwards, the researchers plan to test this peptide in Alzheimer’s mouse models that have increased levels of soluble tau. These mice lose their ability to learn and form new memories around six to eight months old and the team hope that the peptide can prevent or reverse this memory impairment.
“To this end, we must modify PHDP5 so that it can penetrate the blood brain barrier. If this peptide works in these mice models, it can serve as an effective therapeutic tool for Alzheimer’s disease,” said Takahashi.
Related topics
Drug Development, In Vitro, Molecular Targets, Neurons, Neurosciences
Related conditions
Alzheimer’s disease
Related organisations
Okinawa Institute of Science and Technology (OIST)
Related people
Professor Tomoyuki Takahashi