Newly discovered GPCR signalling process opens possibility for novel therapies
Posted: 28 October 2022 | Izzy Wood (Drug Target Review) | No comments yet
US researchers have found that G protein-coupled receptors function in a way not previously known, which suggests untapped potential.
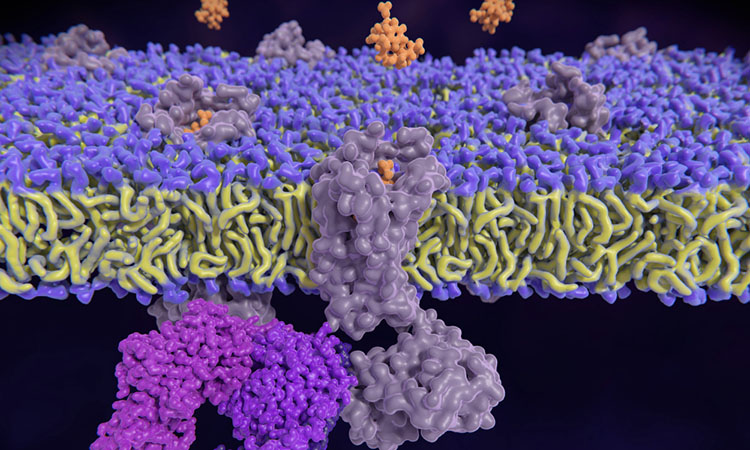

Researchers at University of California (UC), US have identified a new signalling process involving G protein-coupled receptors (GPCRs), a cellular target already exploited by hundreds of diverse drugs. The discovery, published in Nature, opens the possibility of new therapies, including for multiple forms of cancer.
GPCRs are the largest and most diverse group of membrane receptors in eukaryotes. They have become an important therapeutic target for a range of treatments from high blood pressure to asthma to acid reflux. It is estimated that more than one-third of all current US Food and Drug Administration (FDA)-approved drugs target one or more members of the GPCR family.
The canonical view of GPCRs is that they sit on the cell membrane where they activate a variety of reactions, including an enzyme called extracellular-signal-regulated kinase (ERK), which triggers a cascade of signals to control cell growth and survival.
However, the UC researchers found essentially no ERK signalling at the cell plasma membrane. Rather, GPCR-mediated ERK signalling originates at the endosomes — organelles within the cell that sort and help deliver material from the surface to internal destinations or help degrade, recycle and export unneeded material. GPCRs are known to be transported through endosomes.
ERK signalling regulates transcription of genes involved in controlling cell growth. Dysregulation of ERK signalling has been implicated in several pathologies, including cancer. As such, therapeutic intervention targeting members of the ERK signalling pathway is a major endeavour among cancer researchers.
The findings challenge the paradigm that GPCR-regulated ERK signals originate from the cell surface. The data demonstrates that GPCR-mediated ERK signalling originates at endosomes involving hormone-bound and activated receptors.
GPCRs can signal through two distinct classes of molecules: G proteins and arrestins. Arrestins are intracellular proteins involved in terminating plasma membrane signalling. They have been shown to play a major role in ERK signalling but were thought to serve as a scaffold to enable ERK activation.
The new research suggests greater diversity in how GPCRs signal downstream.
“Our data strongly support arrestin’s involvement in ERK activation, but through its capacity to help internalise the receptor and not as a scaffold for ERK as previously thought,” said co-author Dr Roger Sunahara, professor of pharmacology at UC San Diego School of Medicine.
Dr Jin Zhang, professor of Pharmacology at UC San Diego School of Medicine, professor of Chemistry and Biochemistry and professor of Bioengineering at UC San Diego Jacobs of Engineering, added that the new work suggests arrestins and G proteins work collaboratively to activate ERK at the endosomes, with arrestins escorting the receptors to endosomes and G proteins recruiting the ERK activation machinery.
“In terms of translational impact, GPCR drug development has been influenced by concepts like ‘biased signalling,’ with drugs developed to preferentially activate G proteins or arrestins. The discovery that some receptors require the collaboration of arrestins and G proteins to activate ERK is expected to change the way GPCR drugs are developed.”
“We believe these new findings may change the textbook model of GPCR-mediated signalling,” said senior study author Zhang, “and that could have profound implications for future drug development.”
Related topics
Drug Development, GPCRs, Protein, Targets, Therapeutics
Related conditions
Cancer
Related organisations
California University
Related people
Dr Jin Zhang, Dr Roger Sunahara