High-throughput NaV1.9 assay to advance pain therapies
Posted: 14 May 2025 | Drug Target Review | No comments yet
Metrion Biosciences has introduced a breakthrough NaV1.9 screening assay, aimed at overcoming historical challenges in pain research and advancing the development of non-opioid treatments.


Metrion Biosciences, a preclinical contract research organisation (CRO) specialising in ion channel services, has announced the launch of its validated, high-throughput NaV1.9 screening assay. This development has the potential to significantly accelerate the discovery and optimisation of new therapeutics for pain, particularly those targeting non-opioid pathways.
The role of NaV1.9 in pain signalling
NaV1.9 is a voltage-gated sodium channel predominantly expressed in peripheral sensory neurons, which plays a pivotal role in the transmission of pain signals. Mutations in the NaV1.9 channel are linked to extreme pain phenotypes in humans, ranging from severe chronic pain to complete insensitivity. This makes NaV1.9 a strong, yet underexploited target for therapeutic treatment.
Despite its promise, efforts to develop drugs targeting NaV1.9 have been pushed back by the lack of reliable, stable expression systems, limiting the pharmaceutical industry’s ability to study and screen compounds effectively.
Overcoming screening limitations with Metrion’s assay
Metrion’s newly launched NaV1.9 assay addresses these longstanding barriers. Developed in-house using a validated Chinese hamster ovary (CHO) cell line, the assay delivers high reproducibility and low variability, two critical parameters for generating consistent and decision-ready data. It is available with both human and rat NaV1.9 clones, offering insights into species selectivity and supporting the development of more effective, translational therapeutics.
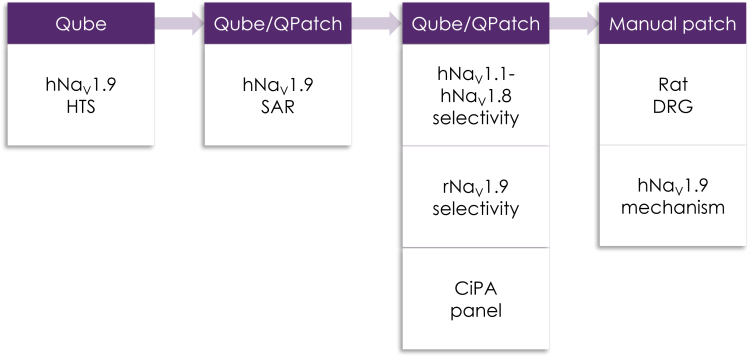

Example of Metrion’s NaV1.9 screening cascade Credit: Metrion Biosciences
An expanded suite for ion channel research
This NaV1.9 assay platform now supports comprehensive selectivity profiling across the full range of pain-related sodium channels, from NaV1.1 to NaV1.9. In addition, Metrion’s services include a wide array of off-target counter screens, cardiac safety evaluations via CiPA panels, and both manual and automated patch clamp technologies. The integration of Qube 384 also allows for rapid, high-sensitivity analysis of extensive compound libraries.
Supporting drug discovery from hit to lead
“The availability of effective assays to study the NaV1.9 sodium channel has been a major stumbling block that has held back development of the next generation of non-opioid pain therapeutics,” said Dr Eddy Stevens, Chief Scientific Officer at Metrion Biosciences.
“Metrion is now able to offer a unique combination of sodium channel expertise, high-throughput screening solutions and research services. These cover the full suite of pain-related sodium channels. By facilitating streamlined compound evaluation and accelerated lead optimisation, this service offering has the potential to bring novel pain therapeutics to market rapidly and more cost-effectively. This important launch represents a major milestone for Metrion, a testament to the dedication and knowledge of our team and reinforces our position as leading the field in ion channel drug discovery,” said Stevens


Dr Eddy Stevens, Chief Scientific Officer, Metrion Biosciences Credit: Metrion Biosciences
Metrion’s NaV1.9 assay offers new hope for the development of non-opioid pain therapeutics. With its validated, high-throughput capabilities and integration into a full suite of ion channel services, the company continues to lead innovation in preclinical drug discovery.
Related topics
Assays, Cell Line Development, Drug Discovery, Drug Discovery Processes, High-Throughput Screening (HTS), Hit-to-Lead, Ion Channels, Neurosciences, Screening, Translational Science
Related conditions
pain
Related organisations
Metrion Biosciences Limited