LVNC congenital heart condition linked to faulty signalling pathway
Posted: 21 October 2019 | Rachael Harper (Drug Target Review) | No comments yet
A new study, which has shown that the congenital heart condition LVNC could be be caused by signalling defects, may lead to potential therapies.

New pre-clinical research from Duke-NUS Medical School in Singapore has shown that the congenital heart condition, left ventricular non-compaction (LVNC) could be caused by signalling defects. These findings could pave the way towards potential diagnosis and therapies.
During normal embryonic heart development, muscle cells migrate and proliferate, forming protrusions inside the cardiac chamber. The projections, called trabeculae, help with heart nutrition and oxygenation until blood vessels form. They eventually dissolve, leading to the formation of a compact, smooth and solid heart wall.
In some embryos, however, the trabeculae fail to dissolve and form a solid heart wall, leading to LVNC, in which the trabeculae project into a thinned lower left heart chamber.
The senior and corresponding author of the study, Assistant Professor Manvendra Singh and his colleagues, wanted to test if a particular protein, Sema3E, is linked to the defects seen in LVNC. Sema3E is known to connect with a cell receptor, called PlexinD1, which is involved in embryonic heart development. However, Sema3E’s existence in the heart had not yet been determined.
The team first turned off Plxnd1, the gene that codes for the PlexinD1 receptor, in a pre-clinical model of embryo hearts. This led to excessive formation of trabeculae that fail to become compacted.
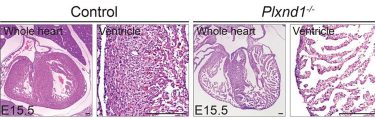

Hematoxylin and eosin (H&E) staining of paraffin sections of E15.5 control heart and transgenic heart with the Plxnd1 gene turned off (credit: Reddemma Sandireddy, Duke-NUS Medical School).
Molecular analyses showed that turning off Plxnd1 led to increased activity from other genes initiated by a ‘Notch signalling pathway’. Inhibiting this pathway partially prevented the hearts from developing malformations. The team also confirmed that many different types of cells in the developing hearts expressed Sema3E. Turning off the gene that codes for Sema3E reduced heart wall thickness.
“Our study helped us to better understand the role of Semaphorin-Plexin signalling in this particular congenital cardiac condition,” said Ms Reddemma Sandireddy, the study’s lead author. “The results indicate that Sema3E connects with its PlexinD1 receptor to turn off the Notch signalling pathway, thus suggesting its role in the formation and maturation of the lower heart chambers.”
Further studies could reveal more information about the processes that regulate heart development and lead to therapies for LVNC and other congenital heart conditions, the researchers say.
The study was published in the journal JCI Insight.
Related topics
Analysis, Drug Targets, Protein, Research & Development, Targets, Therapeutics
Related conditions
left ventricular non-compaction (LVNC)
Related organisations
Duke-NUS Medical School in Singapore
Related people
Assistant Professor Manvendra Singh, Ms Reddemma Sandireddy