Application note: Screening for inhibitors of PD-1 and PD-L1 binding with AlphaLISA technology
Posted: 12 December 2017 | PerkinElmer | No comments yet
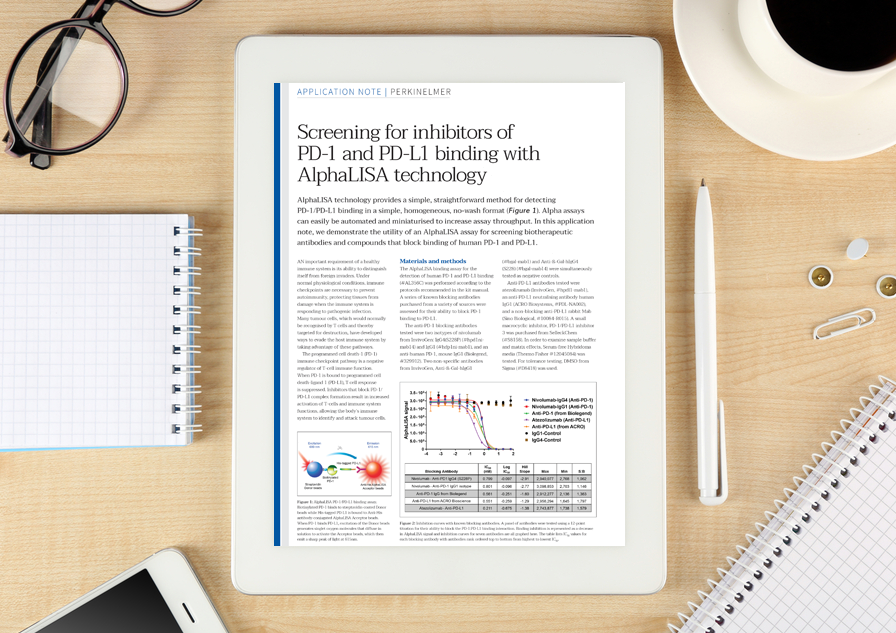
In this application note, PerkinElmer demonstrate the utility of an AlphaLISA assay for screening biotherapeutic antibodies and compounds that block binding of human PD-1 and PD-L1.
AlphaLISA technology provides a simple, straightforward method for detecting PD-1/PD-L1 binding in a simple, homogeneous, no-wash format. Alpha assays can easily be automated and miniaturised to increase assay throughput. In this application note, we demonstrate the utility of an AlphaLISA assay for screening biotherapeutic antibodies and compounds that block binding of human PD-1 and PD-L1.
AN important requirement of a healthy immune system is its ability to distinguish itself from foreign invaders. Under normal physiological conditions, immune checkpoints are necessary to prevent autoimmunity, protecting tissues from damage when the immune system is responding to pathogenic infection. Many tumour cells, which would normally be recognised by T cells and thereby targeted for destruction, have developed ways to evade the host immune system by taking advantage of these pathways.
The programmed cell death-1 (PD-1) immune checkpoint pathway is a negative regulator of T-cell immune function. When PD-1 is bound to programmed cell death-ligand 1 (PD-L1), T cell response is suppressed. Inhibitors that block PD-1/ PD-L1 complex formation result in increased activation of T-cells and immune system functions, allowing the body’s immune system to identify and attack tumour cells.
This application note is restricted - login or subscribe free to access
 Thank you for visiting our website. To access this content in full you'll need to login. It's completely free to subscribe, and in less than a minute you can continue reading. If you've already subscribed, great - just login.
Thank you for visiting our website. To access this content in full you'll need to login. It's completely free to subscribe, and in less than a minute you can continue reading. If you've already subscribed, great - just login.
Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related content from this organisation
- Global high-content screening market set to be worth $2.52bn by 2030
- Assays for protein degradation therapeutics to accelerate undruggable proteome discoveries
- Lab automation market set to increase at CAGR of seven percent
- Brochure: Biologics workflow solutions brochure
- Brochure: Viral research solutions
Related topics
Antibodies, Assays, Screening, T cells
Related organisations
PerkinElmer