New antibody delivery system could improve precision medicine
Posted: 4 January 2022 | Victoria Rees (Drug Target Review) | No comments yet
Scientists have developed a metal-organic framework that, when attached to antibodies, improved their targeted delivery in in vitro studies.
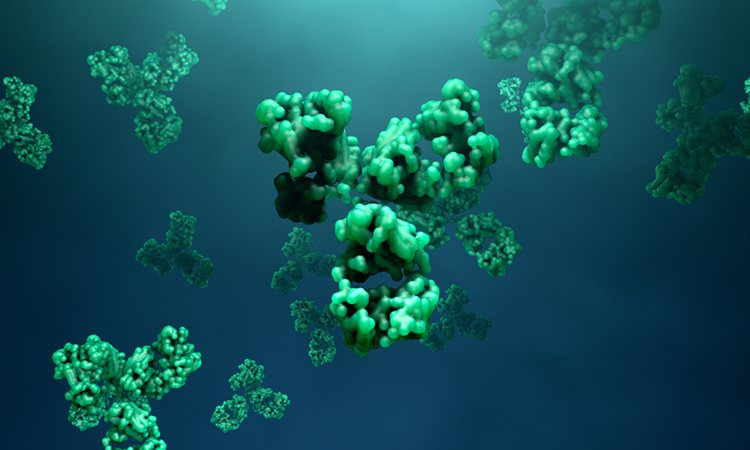
Researchers from Monash University, Australia, in collaboration with the Graz University of Technology, Austria, have developed a synthetic crystal that could be attached to antibodies to supercharge them with potent drugs or imaging agents. According to the scientists, the improved antibodies would then seek out diseased cells with high precision, resulting in fewer adverse effects.
The team developed the world’s first metal-organic framework (MOFs) antibody-drug delivery system that they say has the potential to fast-track potent new therapies for cancer, cardiovascular and autoimmune diseases. The MOF, as outlined in Advanced Materials, is a mixture of zinc and carbonate ions and a small organic molecule: an imidazole, a colourless solid compound that is soluble in water. The researchers say that this not only keeps the payload attached to the antibody, but can also act as a reservoir of personalised therapeutics.
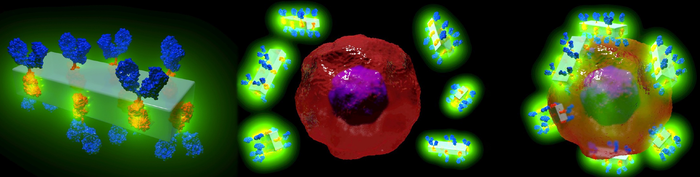
Schematic illustration of the new MOF antibody crystals and their ability to specifically seek out cancer cells to detect them and deliver highly potent drugs with unprecedented precision [credit: Dr Francesco Carraro and Professor Paolo Falcaro (co-first and co-senior author on the Advanced Materials paper)].
An in vitro study showed that when MOF antibody crystals bind to their target cancer cells, if then exposed to the low pH in the cells, they break down, delivering the drugs directly and solely to the desired area.
“The method offers the opportunity to personalise treatment and given the precision possible, may eventually change the current dosage needed for patients, resulting in fewer side effects and making treatments cheaper,” said co-senior author Professor Christoph Hagemeyer, Monash University.
“With just 0.01 percent of chemotherapy currently reaching the cancer tissue, this revolutionary new method can boost the potency of the drugs reaching their target,” said co-first author Dr Karen Alt, Monash University. “With over 80 different monoclonal antibodies approved for clinical use, this approach has enormous potential to improve these antibodies for the targeted delivery of diagnostic agents and therapeutic drugs. The goal is that ultimately the clinical translation of this technology will improve the quality of life for patients suffering from serious diseases.”
ARTICLE: An IgM antibody platform to tackle SARS-CoV-2 and its variants
Related topics
Antibodies, Drug Delivery, Personalised Medicine, Precision Medicine, Proteomics
Related conditions
Cancer
Related organisations
Graz University of Technology, Monash University
Related people
Dr Karen Alt, Professor Christoph Hagemeyer