Kidney fibrosis linked to overactive Hippo signalling pathway
Posted: 1 December 2025 | Drug Target Review | No comments yet
Scientists have used human stem cell–derived kidney organoids to uncover how abnormal Hippo signalling drives scarring in nephronophthisis, a rare genetic kidney disease.

Researchers at Science Tokyo have found a malfunction in a critical cellular signalling pathway that triggers severe kidney scarring in nephronophthisis (NPHP), a hereditary disease that is one of the leading causes of kidney failure in children and young adults. Using advanced human stem cell technology, the researchers have also shown that blocking this pathway can dramatically reduce fibrosis, which could lead to the first effective treatment for the condition.
A devastating disease without a cure
Nephronophthisis is a genetic disorder marked by the gradual accumulation of scar tissue in the kidneys. Over time, this fibrosis destroys healthy tissue, eventually leading to end-stage kidney disease. NPHP accounts for around 10 percent of paediatric dialysis cases, yet patients have no therapeutic options beyond kidney transplantation.
Most cases are caused by mutations or deletions in the NPHP1 gene, which is responsible for producing a protein vital for maintaining normal kidney tubules, called nephrocystin-1. However, efforts to study the disease have been held back by the lack of reliable animal models, as existing ones fail to reproduce the level of scarring seen in human patients.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
Building a human kidney model from stem cells
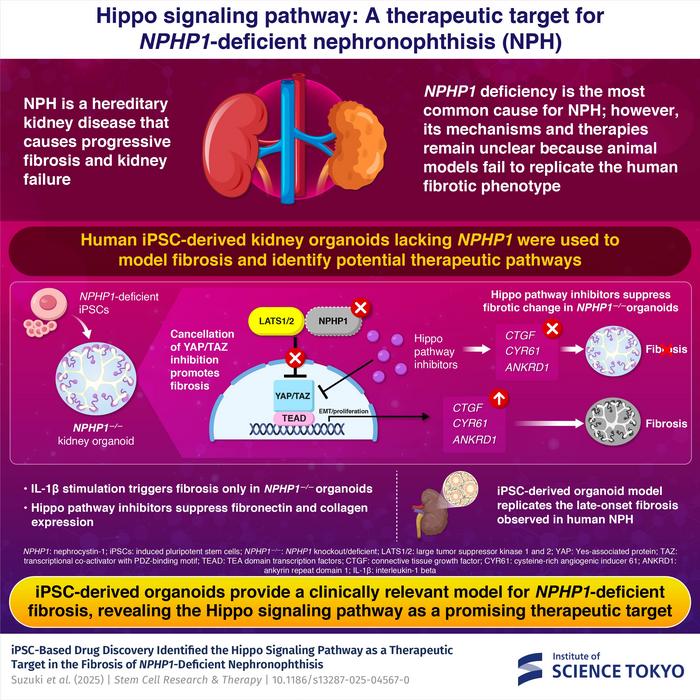
To address this challenge the researchers developed a human kidney organoid model using induced pluripotent stem (iPS) cells. Their work outlined one of the most sophisticated human-based models of NPHP to date.
To address this challenge the researchers developed a human kidney organoid model using induced pluripotent stem (iPS) cells.
“To model the NPHP1 deficiency observed in nephronophthisis, we used genome editing to remove the NPHP1 gene,” explains lead author and Associate Professor Eisei Sohara. “In this way, we generated NPHP1-deficient iPS cell lines which then differentiated into three-dimensional kidney organoids.”
These miniature kidney structures closely mimicked both the cellular composition and architecture of real human nephrons. When exposed to mild inflammatory signals – specifically interleukin-1β – the NPHP1-deficient organoids developed severe fibrotic changes, unlike healthy organoids. Molecular analysis confirmed high levels of fibrosis-related genes including fibronectin, collagen and CTGF.

Researchers used human kidney organoids to uncover how Hippo signaling drives fibrosis and to identify potential drug treatments. Credit: Institute of Science Tokyo
Abnormal Hippo signalling identified as the culprit
Further experiments found that these changes were driven by abnormal activation of the Hippo signalling pathway, an important regulator of tissue repair and organ size. Normally, this pathway helps prevent excessive scarring by controlling YAP and TAZ, two key proteins involved in cell growth.
Further experiments found that these changes were driven by abnormal activation of the Hippo signalling pathway, an important regulator of tissue repair and organ size.
“Our findings reveal that NPHP1 interacts with components of the Hippo pathway to maintain a balance between repair and fibrosis,” says Sohara. “When this interaction is lost, the Hippo pathway becomes overactive, driving progressive kidney damage.”
To test whether blocking this pathway could halt fibrosis, the team trialled several Hippo pathway inhibitors on the organoid model. Among these was verteporfin, a drug already approved to treat macular degeneration.
Verteporfin was found to reverse fibrosis markers and reduce the accumulation of fibrosis-related genes. As verteporfin is already used clinically, it could offer an immediate treatment option for nephronophthisis.
A path toward personalised therapies
This breakthrough marks the first successful drug testing on a human iPSC-derived NPHP model, demonstrating the power of organoid technologies to replace animal models and enable more precise disease research.
Looking ahead, the Science Tokyo team plans to refine their organoid platform to investigate additional signalling pathways and screen new drug candidates. Their goal is to accelerate the development of safer, more effective therapies for kidney fibrosis and other chronic kidney diseases.
Related topics
Disease Research, Drug Development, Drug Discovery, Drug Discovery Processes, Drug Targets, Induced Pluripotent Stem Cells (iPSCs), Molecular Targets, Organoids, Orphan drugs, Regenerative Medicine, Translational Science
Related conditions
Kidney disease, Nephronophthisis (NPHP)
Related organisations
Science Tokyo
Related people
Eisei Sohara (Associate Professor at Science Tokyo)