Researchers reveal structure of SARS-CoV-2 Envelope protein
Posted: 13 November 2020 | Victoria Rees (Drug Target Review) | No comments yet
The molecular structure of the SARS-CoV-2 Envelope protein has been identified by researchers using nuclear magnetic resonance.
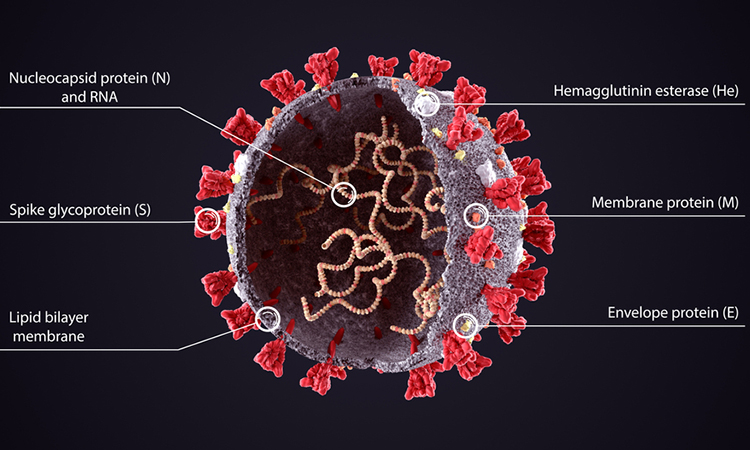

The molecular structure of a protein found on the surface of the SARS-CoV-2 virus has been determined by researchers. The team says that the protein, called the Envelope (E) protein, forms a cation-selective channel and plays a key role in the virus’s ability to replicate itself and stimulate the host cell’s inflammation response. The study was conducted at the Massachusetts Institute of Technology (MIT), US.
The researchers suggest that if ways to block this channel could be devised, then they may be able to reduce the pathogenicity of the virus and interfere with viral replication. Professor Mei Hong, the senior author of the study, said: “Our findings could be useful for medicinal chemists to design alternative small molecules that target this channel with high affinity.”
When the SARS-CoV-2 outbreak began earlier this year, Hong and her students decided to focus their efforts on one of the novel coronavirus proteins. She narrowed in on the E protein partly because it is similar to an influenza protein called the M2 proton channel, which she has previously studied. Both viral proteins are made of bundles of several helical proteins.
“We determined the influenza B M2 structure after about one and a half years of hard work, which taught us how to clone, express and purify a virus membrane protein from scratch and what nuclear magnetic resonance (NMR) experimental strategies to take to solve the structure of a homo-oligomeric helical bundle,” Hong said. “That experience turned out to be the perfect training ground for studying SARS-CoV-2 E.”
The researchers were able to clone and purify the E protein in two and half months. To determine its structure, they embedded it into a lipid bilayer, similar to a cell membrane and then analysed it with NMR, which uses the magnetic properties of atomic nuclei to reveal the structures of the molecules containing those nuclei. They measured the NMR spectra for two months, non-stop, on the highest-field NMR instrument at MIT, a 900-megahertz spectrometer, as well as on 800- and 600-megahertz spectrometers.
The scientists found that the part of the E protein that is embedded in the lipid bilayer, known as the transmembrane domain, assembles into a bundle of five helices. The helices remain largely immobile within this bundle, creating a tight channel that is much more constricted than the influenza M2 channel.
The researchers also found that the SARS-CoV-2 E protein looks nothing like the ion channel proteins of influenza and HIV-1 viruses. In flu viruses, the equivalent M2 protein is much more mobile, while in HIV-1, the equivalent Vpu protein has a much shorter transmembrane helix and a wider pore. How these distinct structural features of E affect its functions in the SARS-CoV-2 virus lifecycle is one of the topics that Hong and her colleagues will study in the future.
They further identified several amino acids at one end of the channel that may attract positively charged ions such as calcium into the channel. They believe that the structure they report in this paper is the closed state of the channel and they now hope to determine the structure of the open state, which should shed light on how the channel opens and closes.
The researchers found that two drugs – amantadine, used to treat influenza and hexamethylene amiloride, used to treat high blood pressure – can block the entrance of the E channel. However, these drugs only bind weakly to the E protein. If stronger inhibitors could be developed, they could be potential drug candidates to treat COVID-19, Hong said.
The study was published in Nature Structural and Molecular Biology.
Related topics
Disease Research, Drug Development, Imaging, Nuclear Magnetic Resonance (NMR), Protein, Proteomics, Research & Development, Targets
Related conditions
Covid-19
Related organisations
Massachusetts Institute of Technology (MIT)
Related people
Professor Mei Hong








